A core principle of modern neurosurgery is the continuous monitoring of brain functions during surgical procedures. This is achieved through intraoperative neuromonitoring (IOM), also known as intraoperative neurophysiology or intraoperative neurophysiological monitoring (IONM). Using advanced neurophysiological techniques, the functions of the nervous system are monitored and protected in real time. The primary goal is to minimize neurological damage during surgery, especially in procedures performed near delicate neurological structures, thereby ensuring the highest possible level of patient safety and well-being.
Intraoperative neurophysiological monitoring (IONM) is a specialty of our clinic. We are one of the leading international clinics in this field, especially in terms of technological equipment, intraoperative methods, experience, number of patients and number of scientific publications. Our clinic has been and continues to be significantly involved in the development of new techniques to avoid neurological deficits worldwide.
Why intraoperative neurophysiological monitoring?
There are two concepts during each operation:
- Achievement of the surgical objective, e.g. radical removal of the tumor
- Avoiding neurological deficits through surgery that reduce the patient's quality of life *, *.
Therefore, every operation must be function-guided *, *, *, *, *, *. The focus is on the following points:
Prevention of nerve damage
IONM can be used to detect changes in nerve function that could indicate impending damage at an early stage.
Support for surgical decision-making
IONM provides real-time information during the procedure to help surgeons access, navigate and perform the operation.
Increased patient safety
The risk of postoperative neurological deficits can be significantly reduced by IONM techniques, as the surgeon can react immediately to potential damage.
Monitoring
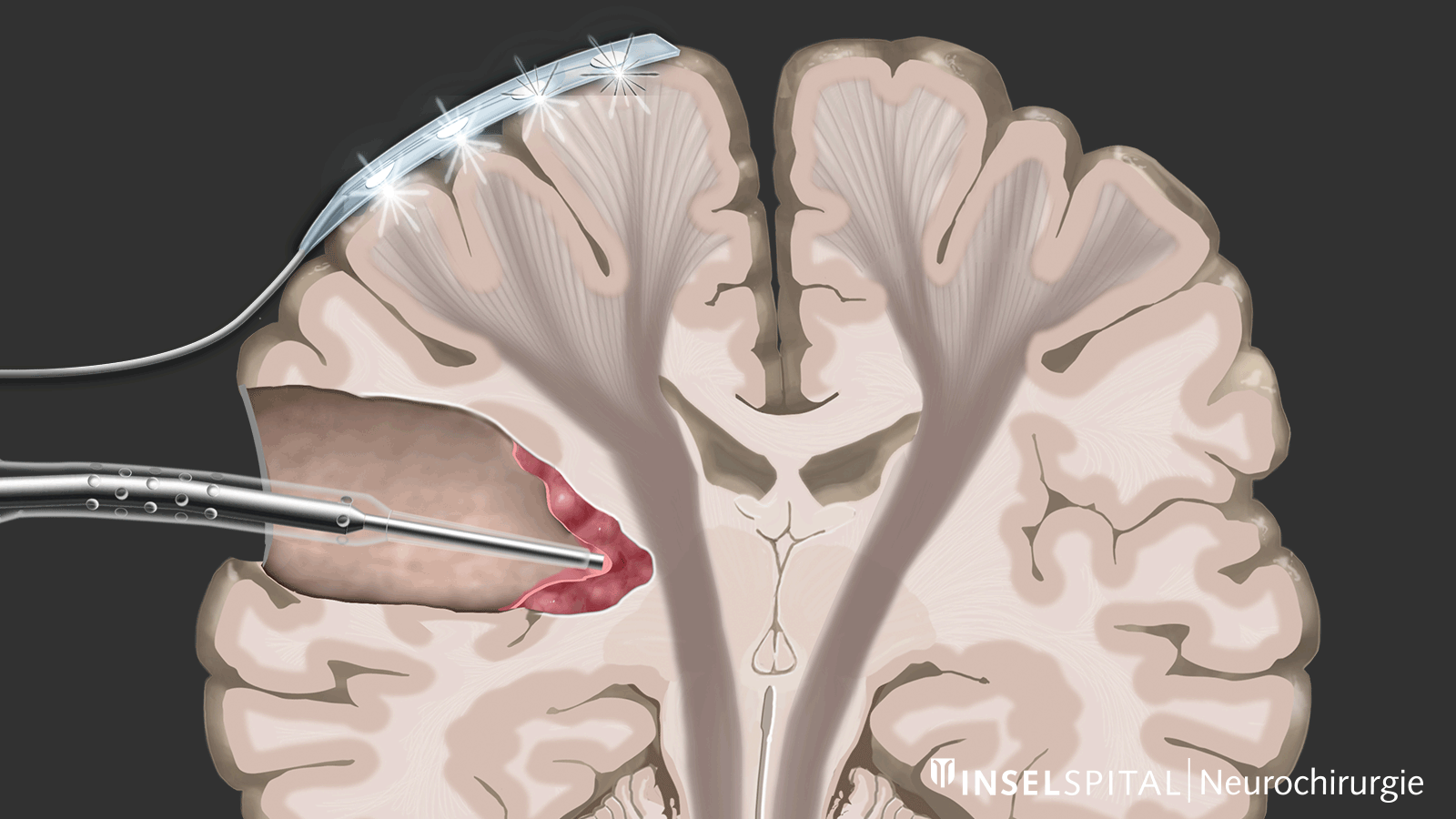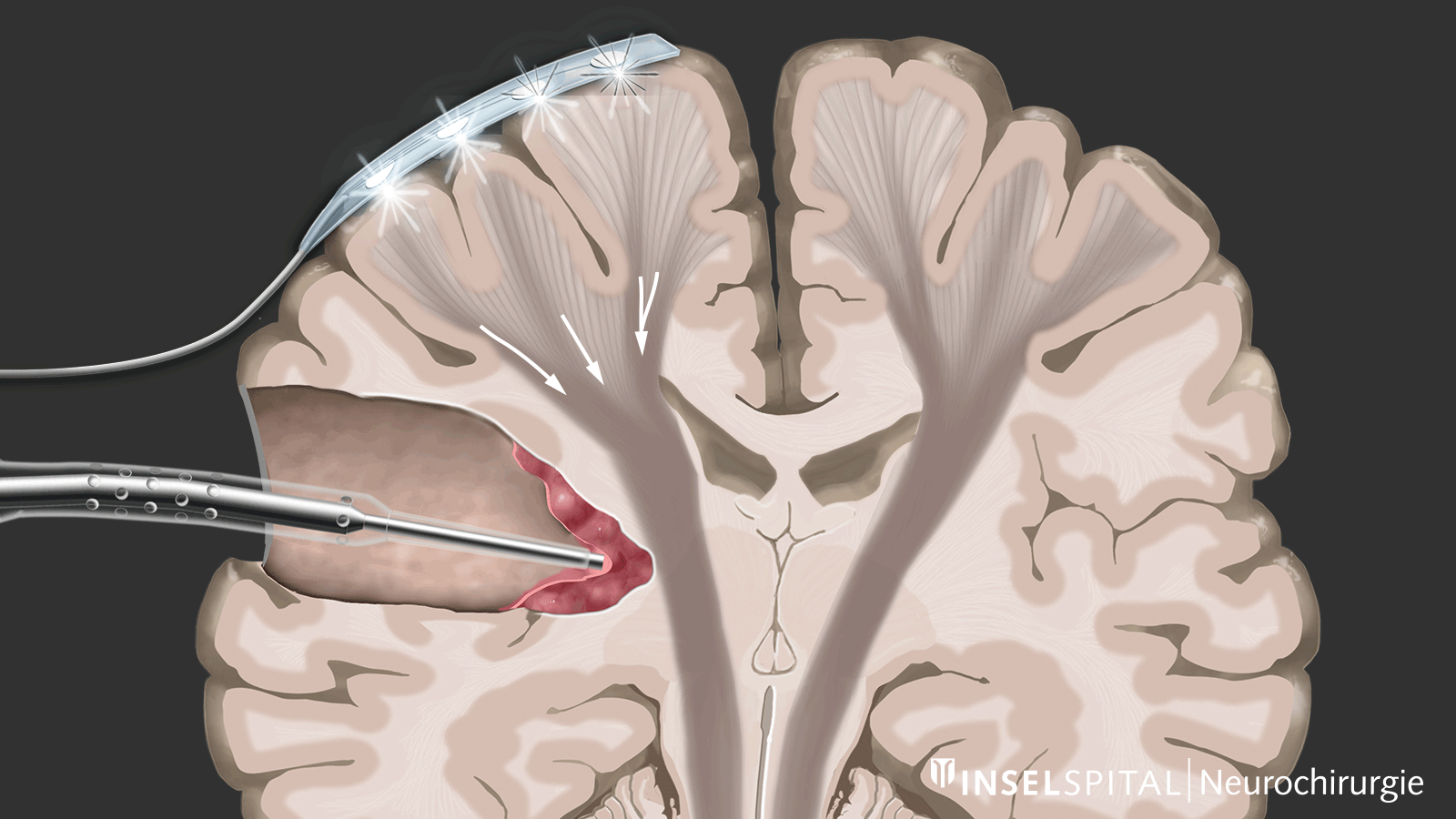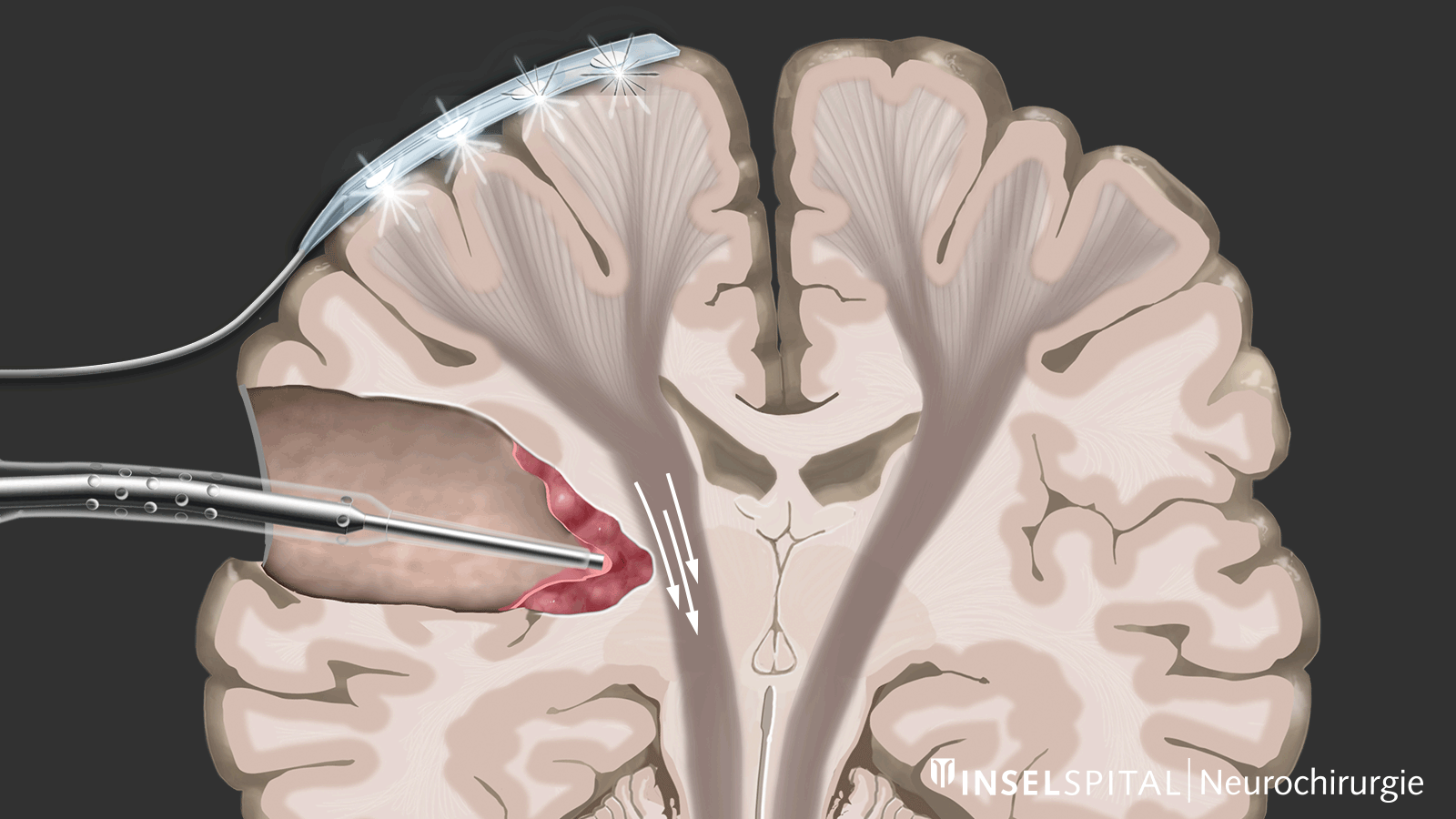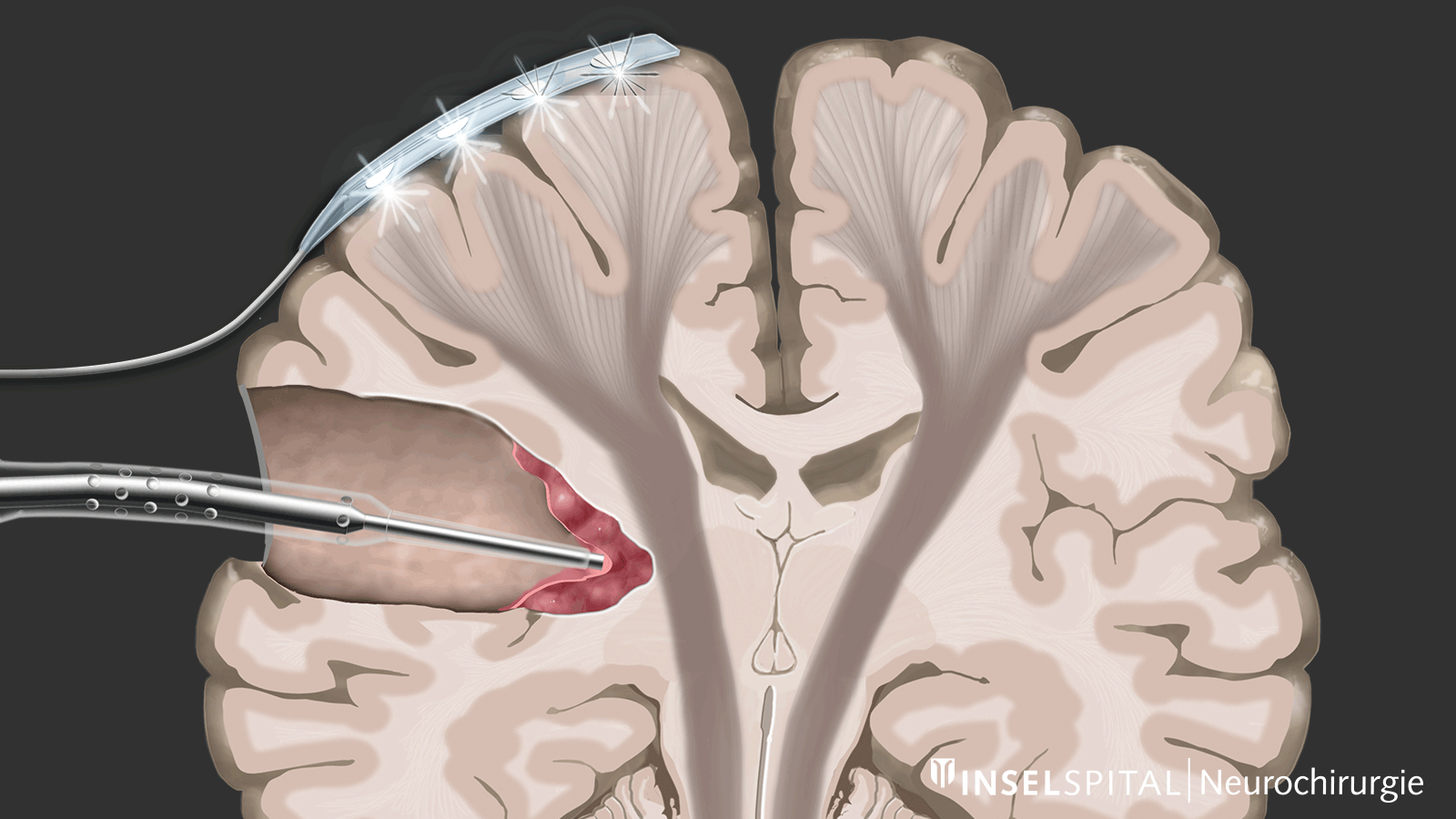
The methods of intraoperative neuromonitoring include, on the one hand, methods that constantly stimulate a known functional site of the brain and nerve tissue during the operation and thus monitor it.
Mapping
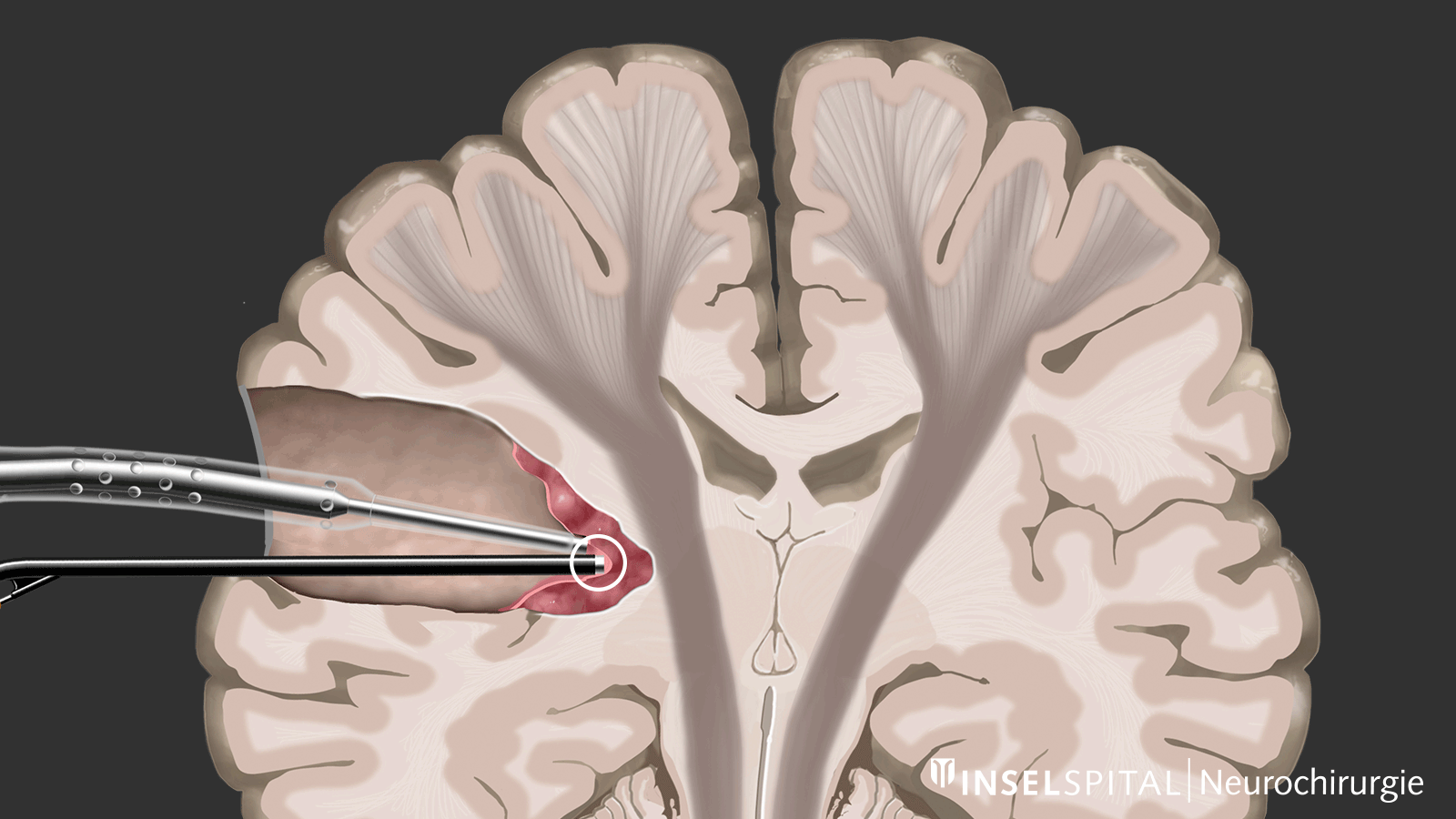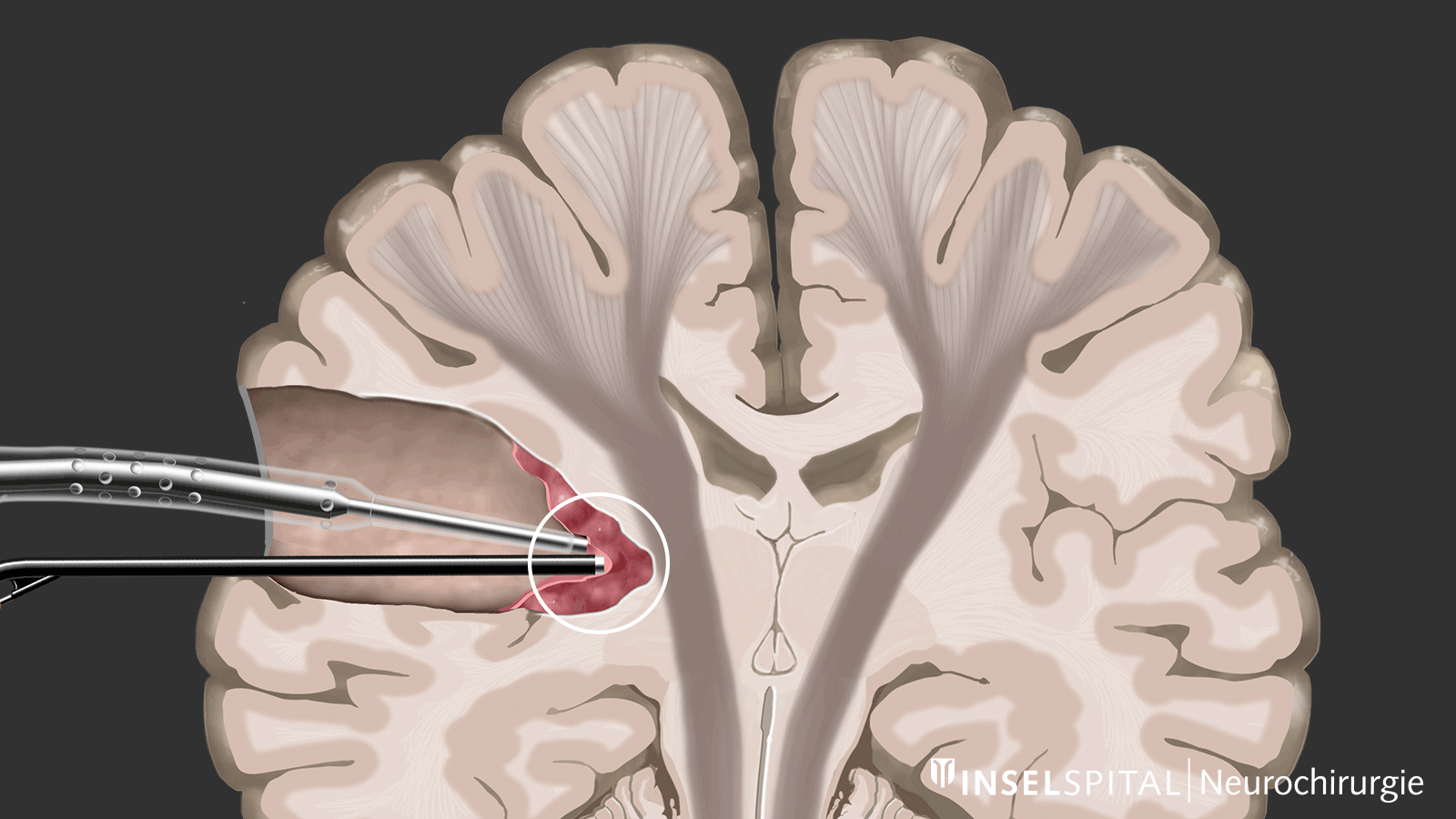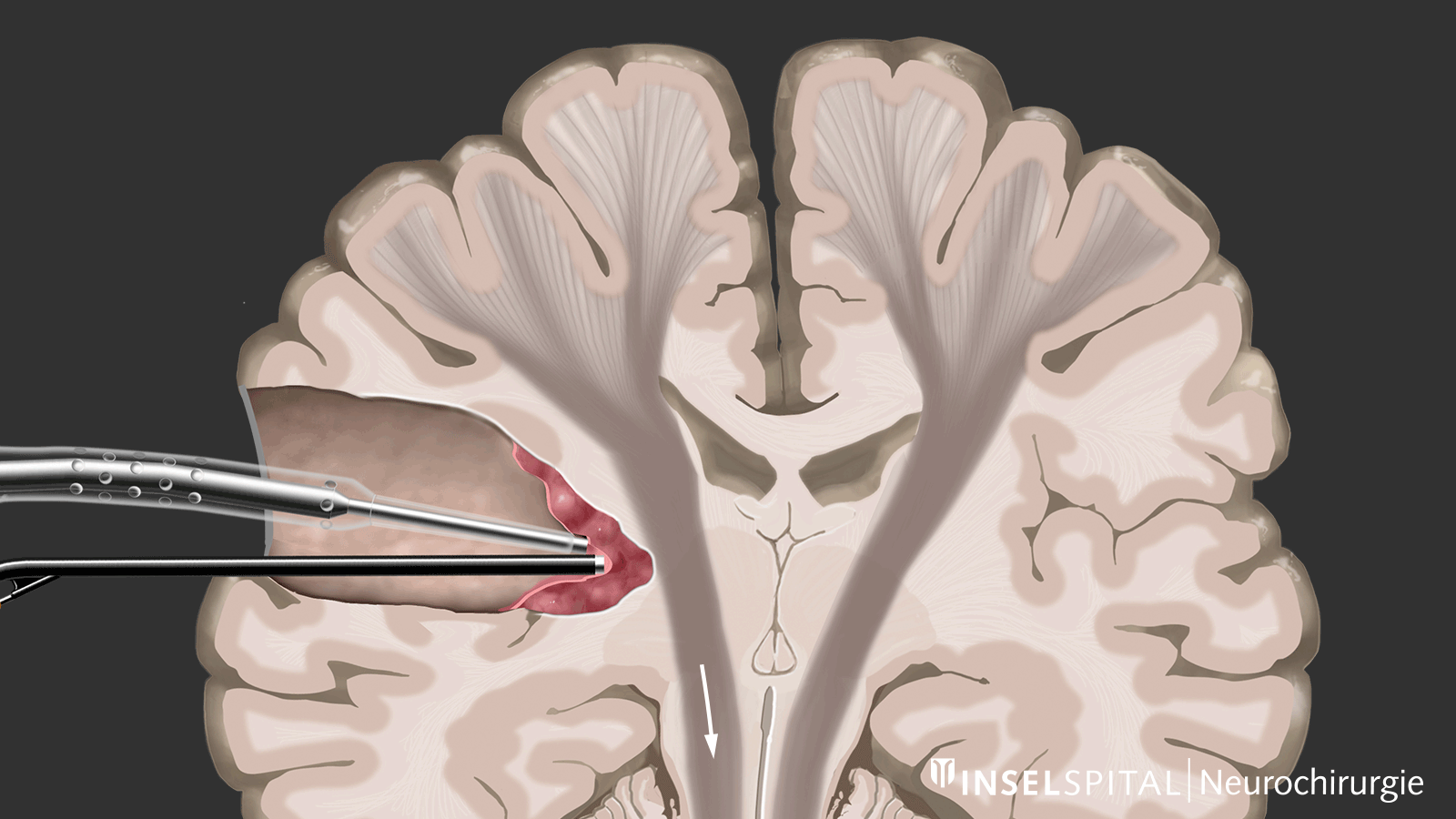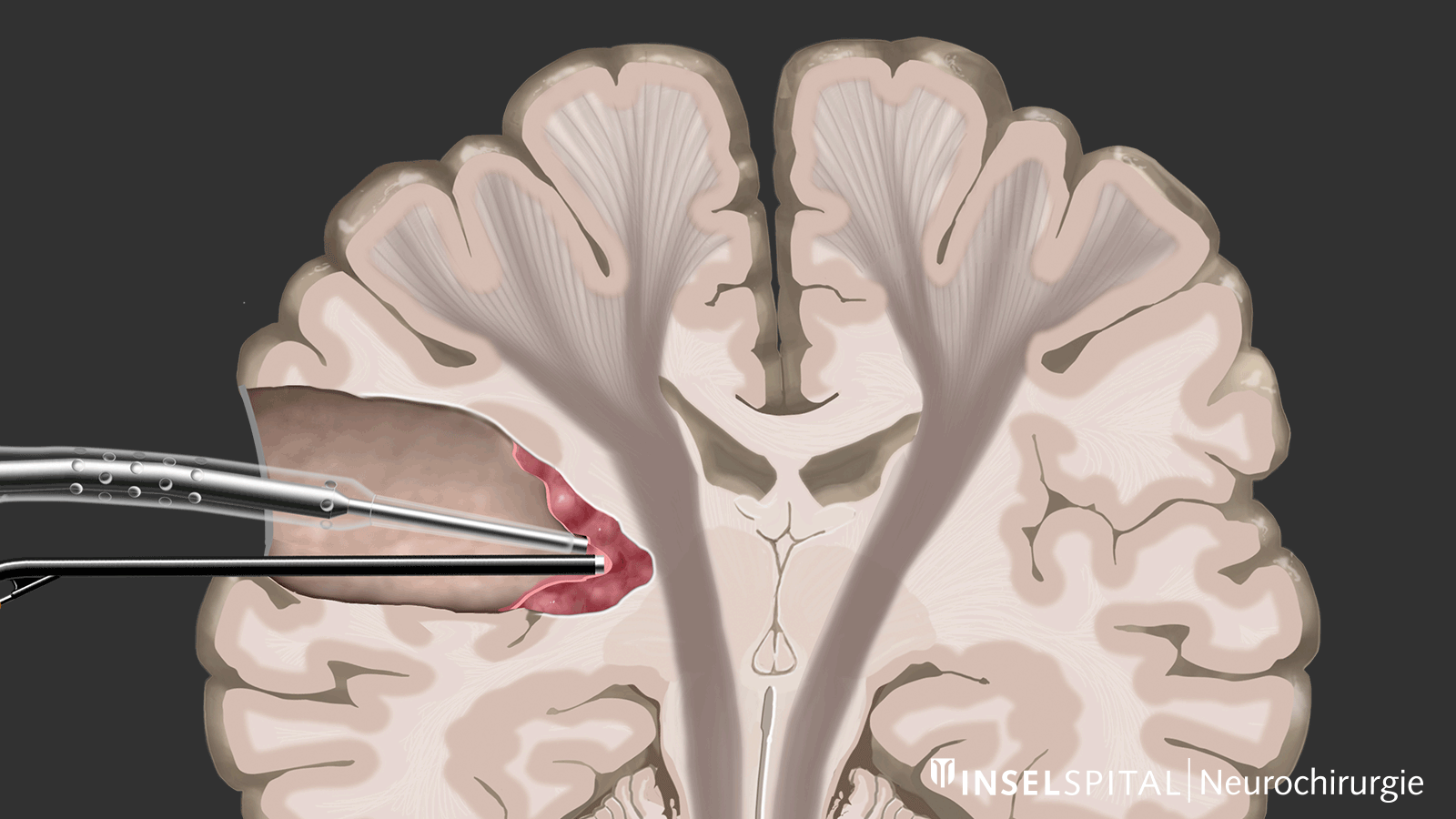
On the other hand, there are methods that use a kind of "search radar" to detect still unknown functional tissue in the vicinity of the surgical field and provide a timely warning of its location, a process called mapping.
Awake brain surgery
Awake brain surgery is also part of the IONM because it makes it possible to monitor the function of critical brain areas in real time by keeping the patient awake and responsive during surgery. This helps to preserve important functions such as speech and movement by directly mapping the corresponding brain regions and avoiding damage.
Brain tumors
A complete or almost complete resection (gross total resection or GTR) remains the gold standard for most intracranial tumor surgeries. Evidence for this exists for glioblastomas as well as for astrocytomas, oligodendrogliomas and other gliomas *, *, *, *, *, *, *.
Whether a brain tumor can be operated on at all depends primarily on its location and proximity to critical brain functions. Based on MRI images, 10–20% of all brain tumors are incorrectly assessed as "eloquent" and inoperable *, *, *. Only intraoperative mapping and monitoring can show in reality whether a tumor previously assessed as inoperable is really inoperable *. In the majority of cases, such a tumor can still be safely operated on using special mapping methods.
Brain aneurysms
When clipping aneurysms, it is important that the large artery remains open while the aneurysm sac is completely clipped – that is, closed. It is known from many studies that this is not perfectly achieved in about 10% of all operations. In these cases, intraoperative neuromonitoring helps to immediately detect critical circulatory disorders. We use monitoring of motor function and sensory perception in almost all aneurysm operations. Both are performed under anesthesia.
Spinal cord tumors
In this type of surgery, the motor pathways are particularly at risk. In most cases, the tumor is located in the immediate vicinity of one or both pathways, and their injury can result in paraplegia. Spinal cord surgery must always be performed with extensive monitoring of motor evoked potentials (MEP), sensory evoked potentials (SEP), D-wave and, if necessary, with mapping of the posterior columns and the pyramidal tract.
Vestibular schwannomas
In vestibular schwannomas, hearing and facial nerve function are of particular importance. The facial nerve is often fused with the tumor capsule. During surgery, 7 different monitoring techniques are used simultaneously.
Brainstem tumors
The nuclei of the cranial nerves and all the pathways that run between the brain and spinal cord are located in the brainstem. Along with the spinal cord, it is the area with the highest concentration of important functions. Surgery in this area requires extensive intraoperative neuromonitoring with up to eight different techniques at the same time.
-
Chang EF, Clark A, Smith JS, et al. Functional mapping-guided resection of low-grade gliomas in eloquent areas of the brain: improvement of long-term survival. Clinical article. Journal of neurosurgery 2011;114(3):566-73.
-
De Witt Hamer PC, Robles SG, Zwinderman AH, et al. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. Journal of clinical oncology. 2012;30(20):2559-65.
-
Raabe A, Beck J, Schucht P, et al. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: evaluation of a new method. Journal of neurosurgery 2014;120(5):1015-24.
-
Bello L, Riva M, Fava E, et al. Tailoring neurophysiological strategies with clinical context enhances resection and safety and expands indications in gliomas involving motor pathways. Neuro-oncology 2014;16(8):1110-28.
-
Sala F, Lanteri P. Brain surgery in motor areas: the invaluable assistance of intraoperative neurophysiological monitoring. Journal of neurosurgical sciences 2003;47(2):79-88.
-
Seidel K, Beck J, Stieglitz L, et al. The warning-sign hierarchy between quantitative subcortical motor mapping and continuous motor evoked potential monitoring during resection of supratentorial brain tumors. Journal of neurosurgery 2013;118(2):287-96.
-
Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. Journal of clinical oncology. 2008;26(8):1338-45.
-
Stummer W, Reulen HJ, Meinel T, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 2008;62(3):564-76; discussion 64-76.
-
Senft C, Bink A, Franz K, et al. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol;12(11):997-1003.
-
Sanai N, Snyder LA, Honea NJ, et al. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas. J Neurosurg;115(4):740-8.
-
McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. Journal of neurosurgery 2009;110(1):156-62.
-
McGirt MJ, Chaichana KL, Attenello FJ, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery 2008;63(4):700-7; author reply 07-8.
-
Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. Journal of neurosurgery 2001;95(2):190-8.
-
De Witt Hamer PC, Robles SG, Zwinderman AH, et al. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol;30(20):2559-65.
-
Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. The lancet oncology 2006;7(5):392-401.
Further reading
Books/book chapters
- Deletis V, Shils J, Sala F, Seidel K. Neurophysiology in Neurosurgery. A modern intraoperative approach. Second edition. Elsevier 2019.
- Deletis V., Seidel K. Intraoperative Neurophysiology during Surgery for Spinal Cord Tumors. In: Arnautović K.I., Gokaslan Z.L. (eds) Spinal Cord Tumors. Springer, Cham. 2019.
Journals
- Seidel K, Beck J, Stieglitz L, Schucht P, Raabe A. Low-threshold monopolar motor mapping for resection of primary motor cortex tumors. Neurosurgery. 2012 Sep;71(1 Suppl Operative):104-14; discussion 114-5.
- Seidel K, Beck J, Stieglitz L, Schucht P, Raabe A. The warning-sign hierarchy between quantitative subcortical motor mapping and continuous motor evoked potential monitoring during resection of supratentorial brain Tumors. J Neurosurg. 2013 Feb;118(2):287-96.
- Raabe A, Beck J, Schucht P, Seidel K. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: evaluation of a new method. J Neurosurg. 2014 May;120(5):1015-24.
- Schucht P, Seidel K, Beck J, Murek M, Jilch A, Wiest R, Fung C, Raabe A. Intraoperative monopolar mapping during 5-ALA-guided resections of glioblastomas adjacent to motor eloquent areas: evaluation of resection rates and neurological outcome. Neurosurg Focus. 2014 Dec;37(6):E16.
- Raabe A, Seidel K. Prevention of ischemic complications during aneurysm surgery. J Neurosurg Sci. 2016 Mar;60(1):95-103.
- Spena G, Schucht P, Seidel K, Rutten GJ, Freyschlag CF, D'Agata F, Costi E, Zappa F, Fontanella M, Fontaine D, Almairac F, Cavallo M, De Bonis P, Conesa G, Foroglou N, Gil-Robles S, Mandonnet E, Martino J, Picht T, Viegas C, Wager M, Pallud J. Brain tumors in eloquent areas: A European multicenter survey of intraoperative mapping techniques, intraoperative seizures occurrence, and antiepileptic drug prophylaxis. Neurosurg Rev. 2017 Apr;40(2):287-298.
- Krieg SM, Lioumis P, Mäkelä JP, Wilenius J, Karhu J, Hannula H, Savolainen P, Lucas CW, Seidel K, Laakso A, Islam M, Vaalto S, Lehtinen H, Vitikainen AM, Tarapore PE, Picht T. Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop Report. Acta Neurochir (Wien). 2017 Jul;159(7):1187-1195.
- Tinkhauser G, Pogosyan A, Debove I, Nowacki A, Shah SA, Seidel K, Tan H, Brittain JS, Petermann K, di Biase L, Oertel M, Pollo C, Brown P, Schuepbach M. Directional local field potentials: A tool to optimize deep brain stimulation. Mov Disord. 2018 Jan;33(1):159-164.
- Deletis V, Seidel K, Sala F, Raabe A, Chudy D, Beck J, Kothbauer KF. Intraoperative identification of the corticospinal tract and dorsal column of the spinal cord by electrical Stimulation. J Neurol Neurosurg Psychiatry. 2018 Feb 7. pii: jnnp-2017-317172.
- Seidel K, Biner MS, Zubak I, Rychen J, Beck J, Raabe A. Continuous dynamic mapping to avoid accidental injury of the facial nerve during surgery for large vestibular schwannomas. Neurosurg Rev. 2018 Oct 26.
- Seidel K, Häni L, Lutz K, Zbinden C, Redmann A, Consuegra A, Raabe A, Schucht P. Postoperative navigated transcranial magnetic stimulation to predict motor recovery after surgery of tumors in motor eloquent areas. Clin Neurophysiol. 2019 Jun;130(6):952-959.
