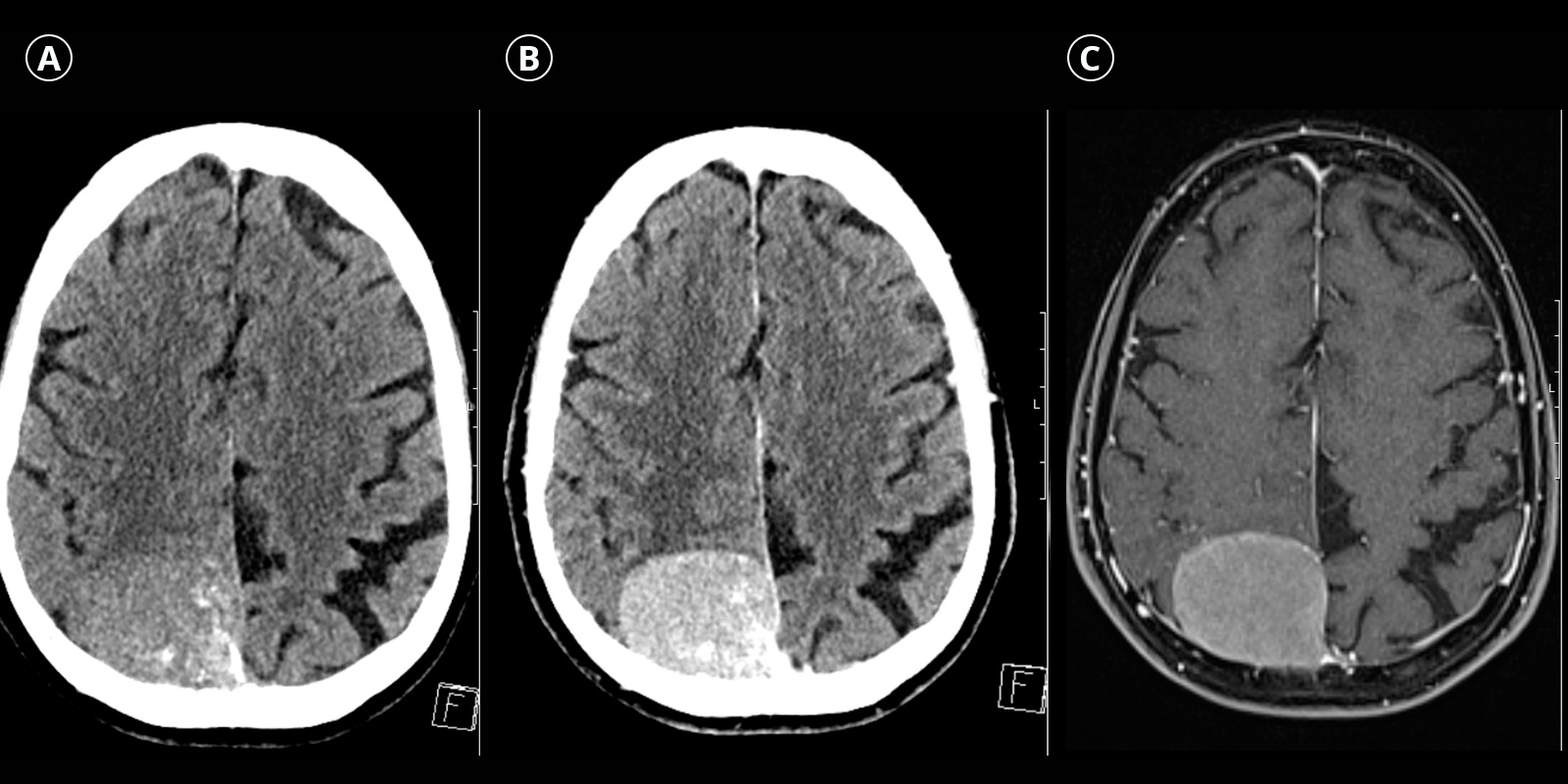
Meningiomas are slow-growing tumors that originate in the meninges. At around 35%, they are among the most common tumors of the central nervous system (CNS) and occur more frequently after the age of 50. More than 90% of meningiomas are benign. When meningiomas grow continuously and reach a size that compresses the brain and causes symptoms, they should be treated.
Clinical significance
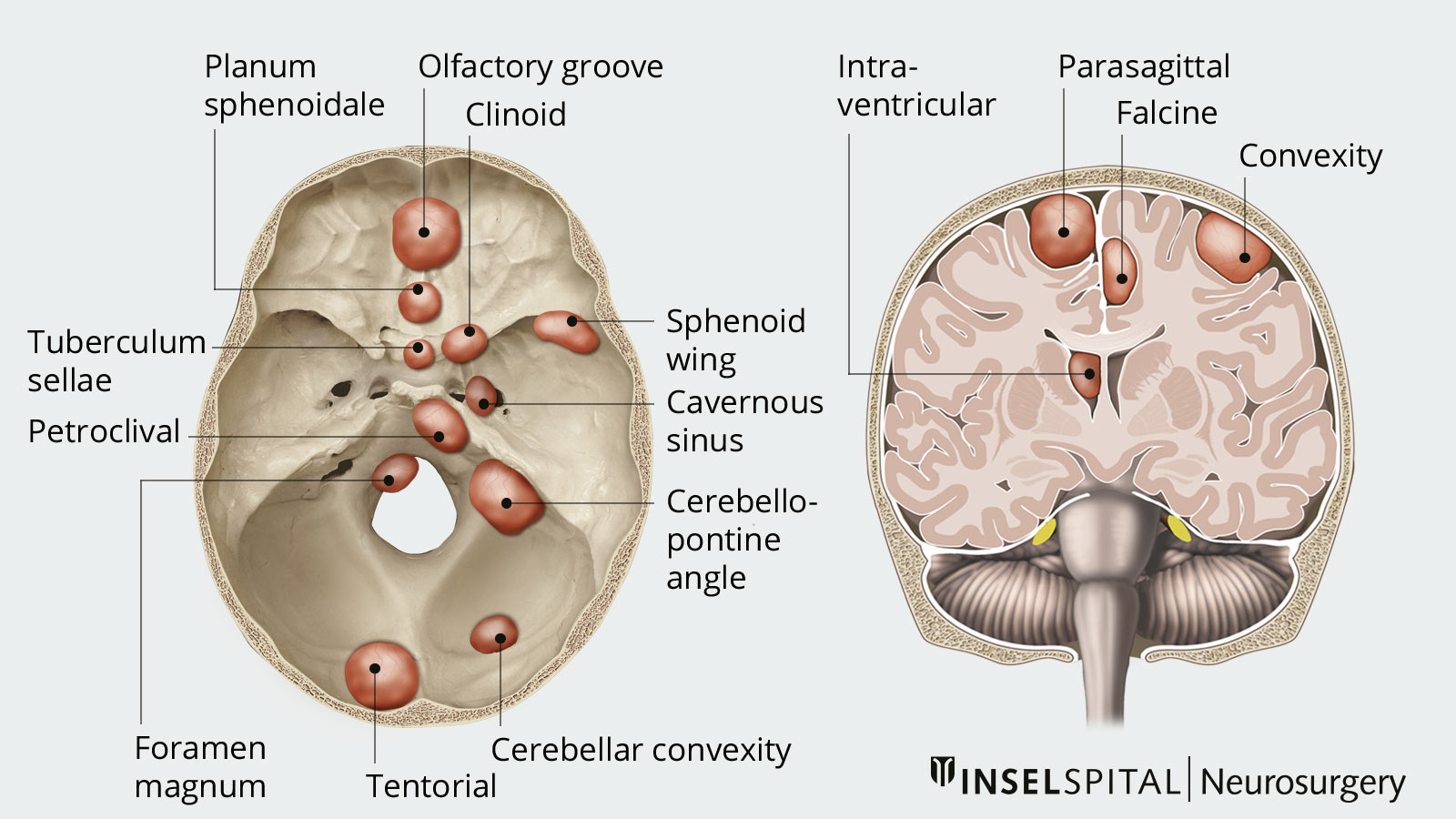
The brain and spinal cord are surrounded by three protective membranes called meninges. Tumors that arise from the cobweb skin (arachnoid membrane) of the meninges are called meningiomas. Meningiomas are the most common benign intracranial tumors. The incidence of disease in the elderly is approximately 3%. Depending on its location, a meningioma causes different symptoms.
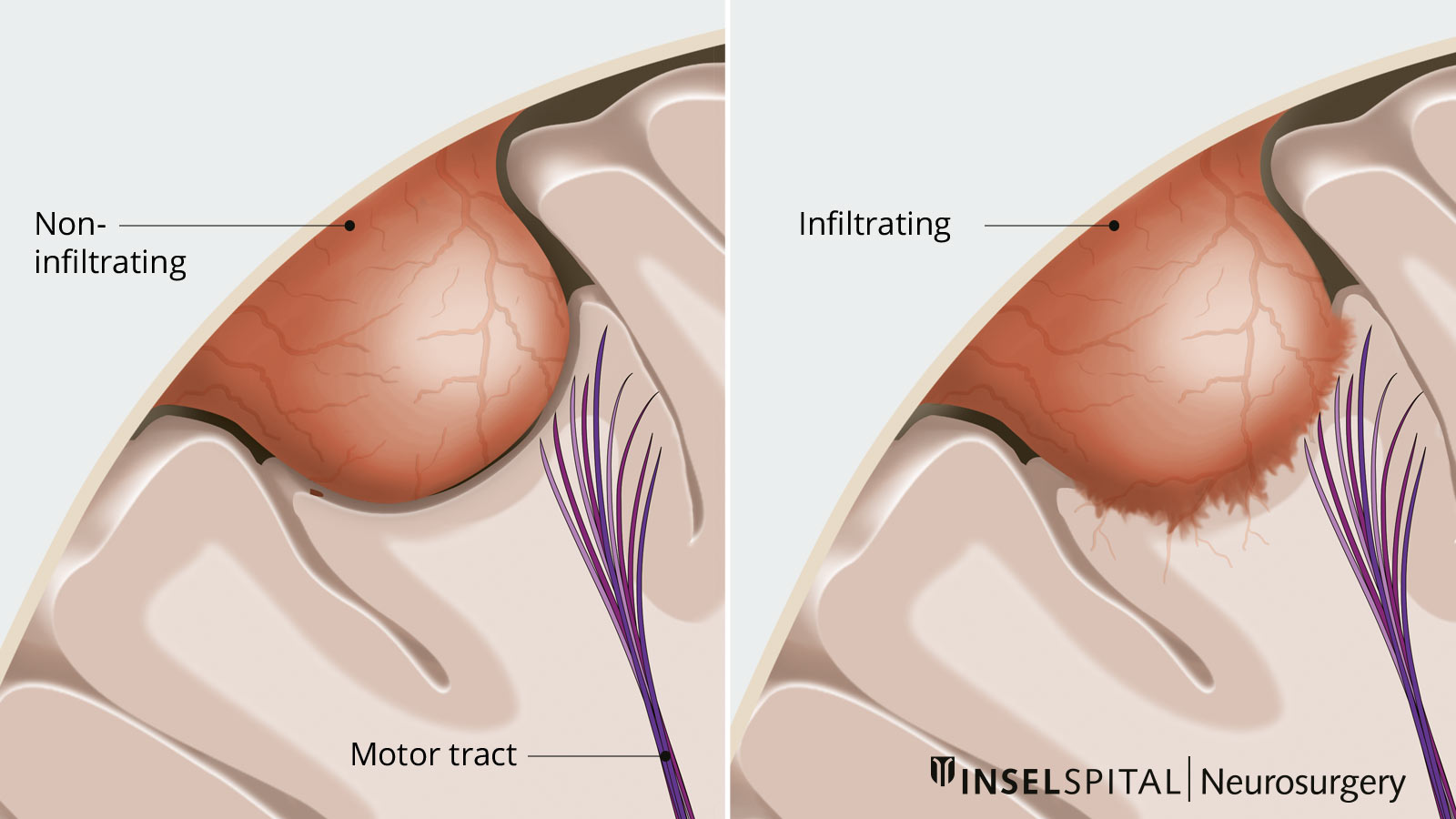
Meningiomas usually grow very slowly, but in rare cases can infiltrate surrounding tissue and metastasize. Although meningiomas are usually benign, they sometimes go undetected for a long period of time due to their insidious, displacing growth, which can lead to severe damage and disability.
What happens if a meningioma is discovered by chance?
Many meningiomas are discovered because patients suffer from general headaches or receive a magnetic resonance imaging (MRI) or a computed tomography (CT) scan after an accident, for example. Such findings are referred to as incidental findings. In most cases, if the meningioma is small, asymptomatic, and not bordering critical areas, the treating physician will opt for follow-up by annual MRI scan. There are indirect signs of growth such as size > 2.5 cm, edema, or a bright cell-rich appearance on the T2 MRI image. In these and other justified cases, an incidentally found meningioma may also be an indication for treatment.
What are the causes of meningiomas?
According to current knowledge, ionizing radiation is the greatest risk factor for the development of meningiomas. Individuals who have been exposed to a particularly high dose of radiation have a significantly increased risk of developing meningioma. These are, for example, atomic bomb survivors (with a 6- to 10-fold increased risk) or patients after radiation therapy in the head and neck area. Patients with the hereditary disease neurofibromatosis type 2 also tend to develop meningiomas.
Frequency and risk factors
- most frequent tumors of the CNS
- increased occurrence from the age of 50 onwards
- biggest risk factor: ionizing radiation
How are meningiomas classified?
According to the World Health Organization (WHO), meningiomas are classified into three grades of severity based on fine tissue criteria:
- WHO grade I = 70%
- WHO grade II = 27%
- WHO grade III = 3%
WHO grade II meningiomas are benign, but grow more rapidly, have an increased tendency to infiltrate surrounding tissue, and often recur after successful therapy (recurrence). WHO grade III meningiomas, on the other hand, are malignant tumors and always require additional radio-chemotherapy.
What symptoms do meningiomas cause?
Meningiomas usually grow slowly, but gradually displace the healthy surrounding brain tissue. Depending on the location and speed of growth, symptoms such as headaches, epileptic seizures, deterioration of vision, smell and speech as well as paralysis and changes in sensitivity may occur. These symptoms typically intensify only gradually due to the slow growth of the tumor.
- headaches
- epileptic seizures
- visual, olfactory and speech disorders
- often only incidental findings
How are meningiomas diagnosed?
Imaging techniques
The diagnosis is usually made in the course of clarifying symptoms or as an incidental finding in the course of other diagnostics.
Both computed tomography (CT) and magnetic resonance imaging (MRI) show meningiomas as well-defined, extra-axial (primarily located outside the brain tissue) tumors that lie broadly on the hard meninges (dura mater) and compress the adjacent brain tissue. When contrast medium is administered, meningiomas absorb it homogeneously. In CT, calcifications as well as secondary bone changes can be detected as an additional feature. MRI often shows outwardly extending dural thickenings, the so-called dural tail sign.
How are meningiomas treated?
Small meningiomas that do not increase in size can be treated in a conservative manner. If meningiomas grow continuously or have reached a size that compresses the brain, causes cerebral edema, or becomes symptomatic, they should be treated. Standard therapy is surgery, less common is radiosurgical therapy. Because of the variable location and size, the decision about the best approach must always be discussed on an individual basis. The optimal treatment therefore depends on the size, location and growth rate of the tumor, as well as on histopathological tissue analysis and the patient's general condition.
Brain Tumor Center
This weekly tumor board is composed of specialists from neurosurgery, neurology, neuro-oncology, nuclear medicine, radio-oncology as well as pathology.
At Inselspital, the best possible treatment strategy is determined individually for each patient. This is done at the certified Brain Tumor Center, where an interdisciplinary team discusses and determines all treatment options individually for each patient.
This weekly tumor board is composed of specialists from neurosurgery, neurology, neuro-oncology, nuclear medicine, radio-oncology as well as pathology.
Conservative treatment
Patients with asymptomatic incidental findings, small meningiomas or older age are initially treated conservatively. This means that clinical controls including imaging are performed regularly to monitor the progression of the disease. Initial follow-up is performed within 3‒6 months after the first diagnosis. If the findings are stable, the subsequent time intervals between follow-up examinations can be progressively extended.
In various studies, stable meningioma size without growth tendency was observed in about 50‒70% of patients during imaging follow-up over an average period of 2‒5 years. If the meningiomas increase in size or new symptoms develop, a new decision on further therapy should be made.
Operation
The primary therapy for a symptomatic, enlarging meningioma is complete microsurgical removal. If this is possible, it means cure for the patient in most cases. Therefore, the top priority of treatment is to remove the tumor as gently as possible while preserving its function and maintaining a high cosmetic standard.
In this procedure, the meningioma is first reduced in size from the inside so that the border to the neighboring tissue is relieved. Then the tumor is carefully dissected away from the surrounding normal brain tissue under the microscope. The tumor-bearing meninges are usually removed and replaced as well. The interlocking between the normal brain and the meningioma varies in intensity from patient to patient. Some of the tumors have a smooth border, others have infiltrated the brain and are supplied with blood by normal brain vessels.
In these cases, if an important brain area is affected, the operation must be performed under functional monitoring. For this purpose, the latest technical procedure such as neuronavigation with augmented reality and intraoperative neuromonitoring, are used. They enable the neurosurgeon to work with maximum precision and safety under the operating microscope and to make the patient transparent, i.e. to look into tissue.
Preoperative embolization
Meningiomas are usually tumors with a good blood supply. Therefore, it is very important that the corresponding blood supply via so-called feeders is quickly cut during surgery. In selected cases, for example in certain skull base meningiomas, the arterial tumor feeder cannot be reached quickly, so that preoperative endovascular embolization of the tumor can be used. This artificial occlusion of the blood vessels prior to surgery can increase surgical safety in selected cases.
Irradiation
In patients with meningioma that is inoperable or only partially operable, radiation therapy is a treatment option with good tumor size control. Radiation therapy is also used as an adjuvant for higher-grade meningiomas or incomplete resection. However, the meningiomas must not have exceeded a certain size for this.
Radionuclide therapy
In difficult cases with gradual disease progression despite surgery and radiation, radionuclide therapy offers another therapeutic option. This involves targeting the tumors with radioactive drugs. The radiopharmaceutical, the radioactive substance, binds to special receptors on the surface of the meningioma, the somatostatin receptors, and achieves a local radiation effect there, thus destroying the tumor cells.
Therapy complications
The complication rate of surgical tumor removal and possible further irradiation is influenced by several factors such as size, location and accessibility of the tumor as well as the clinical condition and age of the patient. Location and accessibility of the tumor as well as the clinical condition and age of the patient. In general, the risk of severe complications in "normal" meningioma surgery is less than 2%. It is higher for complicated tumors. We will be happy to discuss your individual risk profile in person based on your MRI results.
What do future courses look like?
The prognosis depends primarily on the location, size and histological nature of the tumor (WHO grade I‒III). An important factor here is also whether the tumor could be completely or only partially removed. Depending on the extent of surgical removal and the results of histological analysis, the following procedure is recommended as a guideline and in individual cases depending on molecular biomarkers:
WHO grade I and total resection
One control after surgery, depending on the risk, another control after 2.5 and 10 years
WHO grade I and incomplete resection
Annual follow-up and/or radiosurgery
WHO grade II and total resection
Follow-up controls with MRI every 6‒24 months, follow-up irradiation if necessary
WHO grade II and incomplete resection
Annual follow-up and/or radiosurgery
WHO grade III
Follow-up irradation, radionuclide therapy or experimental chemotherapy if necessary, and follow-up controls with MRI every 3‒6 months