Parkinson's disease is one of the most common neurological disorders. Around 6.3 million people are thought to be affected worldwide. Patients primarily suffer from disturbances in the course of movement and mobility, a disturbing tremor at rest, and gait disturbances. To alleviate the suffering of this disease, it is very important to tailor the therapy to the individual needs of the patient. In the case of deep brain stimulation (DBS), we routinely perform this at Inselspital under general anesthesia. This requires a high level of expertise and a great deal of experience on the part of the treating physicians.
Who is affected by Parkinson's disease?
There are approximately 15,000 Parkinson's patients in Switzerland. Every year, about 3000 patients in Switzerland develop Parkinson's disease. Men are affected slightly more often than women. The average age of onset is between 65 and 70 years.
What are the causes of Parkinson’s disease?
In most cases, Parkinson's disease is thought to be caused by a combination of external environmental influences and hereditary factors. In rare cases (5–10% of Parkinson's patients), the disease is genetic. Mutations in certain genes (PARK 1-16) are inherited and can lead to Parkinson's disease. This form is called monogenetic variant. Affected patients usually develop the disease before the age of 40.
Parkinson's is a neurodegenerative disease characterized by the microscopic progressive destruction of nerve cells in certain regions of the brain. Particularly affected is the substantia nigra, a nuclear structure in the area of the midbrain, where the messenger substance dopamine, a so-called neurotransmitter, is formed. The dopamine signal from the substantia nigra is essential for behavioral control. It regulates information processing in the basal ganglia – a collection of nuclei deep in the cerebrum – which play a central role in behavioral planning as well as selection and motor control. Simply put, the dopamine signal triggers certain activities while inhibiting others, allowing for coordinated behavior. In addition, dopamine is important for motor learning and motivational control.
As the disease progresses, nerve cells in other regions of the brain that do not produce dopamine are also affected. This results in numerous symptoms (vegetative disorders, pain, sleep disturbances, mental disorders) that become increasingly burdensome for the patient as the disease progresses. One example is the pedunculopontine nucleus in the brainstem, which plays important roles in controlling stable walking and regulating attention and sleep.
What are typical symptoms of Parkinson's disease?
The cardinal symptom of Parkinson's disease is the impairment of movement functions, the slowing down of movement up to immobility (bradykinesis, hypokinesis, akinesis). Other main symptoms can be added in individually varying degrees:
- Tremors (Tremor)
- Muscle stiffness (Rigor)
- Postural instability with tendency to fall
In addition, non-motor symptoms may occur as part of Parkinson's disease:
- Vegetative symptom
- Psychological symptoms
- Cognitive symptoms
Bradykinesis, hypokinesis, akinesis
Bradykinesis is a retardation of voluntary motor activity. Affected patients are less able to switch quickly between different motor tasks or movements, and activities are generally slowed. Practically, gait patterns become small-stepped, handwriting smaller and less sweeping, speech softer, swallowing less frequent, etc. At first, patients notice this during activities with their hands, such as writing or playing a musical instrument. This symptomatology increases as the disease progresses.
The lack of movement or reduced mobility is called hypokinesis.
A high degree of symptoms up to immobility is called akinesis.
Tremors
Tremors characteristically are resting tremors of the extremities – tremors without voluntary movement during physical relaxation. The typical rhythmic tremor movement of the fingers resembles the finger movement during pill turning and is therefore often referred to as pill-rolling tremor. The tremor decreases as soon as the affected limbs are moved purposefully. Typically, the tremor is intensified during emotional or mental exertion.
Rigor
Parkinson's syndrome permanently increases muscle tone throughout the entire movement process. Due to this muscle stiffness, patients have the impression that their limbs are "paralyzed" and also often suffer from painful cramps.
Postural instability
Walking upright and balance are impaired. Maintaining a straight posture becomes increasingly difficult as the disease progresses. For this reason, falls are a possible dangerous consequence of Parkinson's disease.
Freezing of gait
Patients may also suffer from a specific gait disorder known as "freezing of gait". In this case, walking Parkinson's patients suddenly and without warning experience a blockage. The affected person appears frozen or paralyzed. Due to the hunched-over posture of most Parkinson's patients, loss of balance and falls can also occur *.
Non-motor symptoms
Although Parkinson's is considered a classic movement disorder and motor symptoms are the primary concern for patients, there are other non-motor symptoms associated with Parkinson's:
- Vegetative disorders: dysfunction during urination, blood pressure disorders, temperature regulation disorders, sleep disorders.
- Psychological disorders:depression, anxiety
- Cognitive disorders: behavioral disorders, dementia
- Sensory disorders: visual disturbances, olfactory disturbances, sensory disturbances.
Cognitive difficulties often manifest in the advanced stages of the disease and, in the worst case, end in dementia. This Parkinson's dementia differs clinically from the probably best-known form of dementia, Alzheimer's dementia. While Alzheimer's dementia is primarily characterized by memory disorders, Parkinson's dementia is primarily characterized by behavioral abnormalities such as apraxia. This means that the affected patient can no longer correctly perform complex movements, such as making coffee, dressing, etc.
What are the different forms of Parkinson's disease?
Depending on which main symptoms are particularly pronounced, a distinction is made between different types of Parkinson's disease:
- Akinetic-rigid type: lack or slowness of movement and rigor are prominent
- Tremor-dominant type: pronounced tremors, less rigor and postural instability
- Equivalent type: the classic main symptoms akinesia, rigor and tremors are approximately equally pronounced
Symptoms of Parkinson's disease can also occur in the context of other diseases of the nervous system. The prognosis is different here, and the affected patients also do not respond or only respond inadequately to the usual therapies (drug treatment or neuromodulation). The diagnosis of these atypical Parkinson's syndromes is therefore important both prognostically and therapeutically.
Atypical Parkinson's syndromes are:
- Multiple system atrophies (MSA)
- Lewy body dementia (LBD)
- Progressive supranuclear palsy (PSP)
- Corticobasal degeneration (CBD)
How is Parkinson's disease diagnosed?
The diagnosis of Parkinson's disease is made clinically. The patient’s medical history and the clinical-neurological examination findings are of central importance. Individual symptoms can be clinically classified and are used for staging. This so-called staging is done by means of the Hoehn and Yahr scale (Hoehn 1967) and the Unified Parkinson's Disease Rating Scale (UPDRS). Additional examinations such as magnetic resonance imaging (MRI), dopamine transporter scintigraphy (DAT scan), cerebrospinal fluid (CSF) diagnostics, etc. are only used as supportive measures to rule out non-idiopathic Parkinson's syndromes for which the cause is known.
Which treatment options are available for Parkinson's patients?
Close collaboration between several specialist disciplines is crucial for the successful treatment of Parkinson's disease.
Drug therapy is the primary treatment and is carried out by neurologists with special knowledge in the field of movement disorders.
Surgical procedures can play an important supportive role in therapy. Common procedures include:
- lesional procedures such as MR-guided focused ultrasound
or
- non-lesional procedures such as deep brain stimulation (DBS)
These procedures are highly specialized and require extensive training in functional neurosurgery. The choice of procedure depends primarily on medical factors and the individual needs of the patient and should always be discussed in detail with our specialists. The close interdisciplinary collaboration between neurologists and functional neurosurgeons at Inselspital ensures that each of our patients receives competent and, above all, individually tailored holistic treatment.
Drug therapy
Due to the destruction of the nerve cells of the substantia nigra, which release dopamine as a neurotransmitter, there is an imbalance in the finely tuned transmitter system of the basal ganglia. As a result, in very simplified terms, there is a deficiency of dopamine and an excess of acetylcholine in the signal transmission between the nerve cells in the area of the basal ganglia. Drug treatment is primarily aimed at restoring dopaminergic signal transmission and simultaneously inhibiting acetylcholine transmission. Most often, the following drugs are used for this purpose:
L-Dopa
L-dopa is a precursor to dopamine and is converted into dopamine in the brain by the enzyme DOPA decarboxylase (DDC). L-dopa is the most effective and well-tolerated drug for the treatment of Parkinson's symptoms and is currently the drug of choice. It mainly improves akinesia and rigor, less so tremor. Long-term observations have shown that L-DOPA therapy over several years leads to a gradual decline in efficacy and that the effect is subject to significant fluctuations (so-called fluctuations in efficacy) during the course of the day.
Dopamine agonists
Dopaminergic agonists such as pramipexole, ropinirole, rotigotine, piribedil act directly on dopamine receptors. These are used, among other things, to decrease the amount of L-DOPA needed and in cases where patients no longer respond reliably to L-DOPA. Advantages of dopamine agonists include having fewer fluctuations in effect as well as less hypermobility (so-called dyskinesia) as side effects. Disadvantages are that behavioral abnormalities such as obsessive-compulsive disorder or reduced impulse control have been observed in 15% of patients during treatment. This is expressed, for example, in gambling addiction, spending sprees, money problems, hypersexuality, etc.
Monoamine oxidase type B inhibitors (MAO-B)
MAO-B (rasagiline) has a minor dopaminergic effect by inhibiting dopamine degradation. They are mostly used as an additive agent to enhance the effect of L-DOPA and to reduce the fluctuations in the effect of L-DOPA therapy.
Anticholinergics
An example is benserazide. This longest-known therapy inhibits acetylcholine signaling. These drugs continue to have importance in cases where tremor or vegetative disorders are the dominant symptoms. The effect on akinesia and rigor is in the background. Possible side effects include memory impairment and may be a problem in advanced Parkinson's.
The list of medications listed here does not claim to be complete. These drugs can be used individually or in combination.
Injection therapy
In addition to the drugs listed, the dopamine agonist apomorphine can also be used as an injection under the skin or in the form of an apomorphine pump for continuous administration into the duodenum. This therapy comes into play when drug treatment with tablets no longer helps sufficiently.
Surgical therapy
The surgical treatment of Parkinson's disease is a subspecialty of functional neurosurgery that dates back to pioneering work by Alim Louis Benabid in the 1990s. His research showed that the implantation of electrodes for high-frequency stimulation in specific brain regions can achieve functional improvements, while side effects can be effectively avoided by precisely adjusting the stimulation intensity *.
This novel treatment was named deep brain stimulation (DBS) and is now the standard surgical therapy at Inselspital*.
There are three areas in the brain that can be targets for DBS in patients with Parkinson’s disease:
- Subthalamic nucleus (STN)
- Globous pallidus internus (GPi)
- Ventral intermediate nucleus of the thalamus (VIM)
These possible target areas must be discussed as alternatives in each patient individually in the expert board. Stimulation in the subthalamic nucleus (STN) is the target of choice, because it can successfully treat akinesia and rigor and the dosage of medication can usually be considerably reduced. A potential disadvantage is the worsening of pre-existing postural instability. According to studies, GPi stimulation * is currently equivalent.
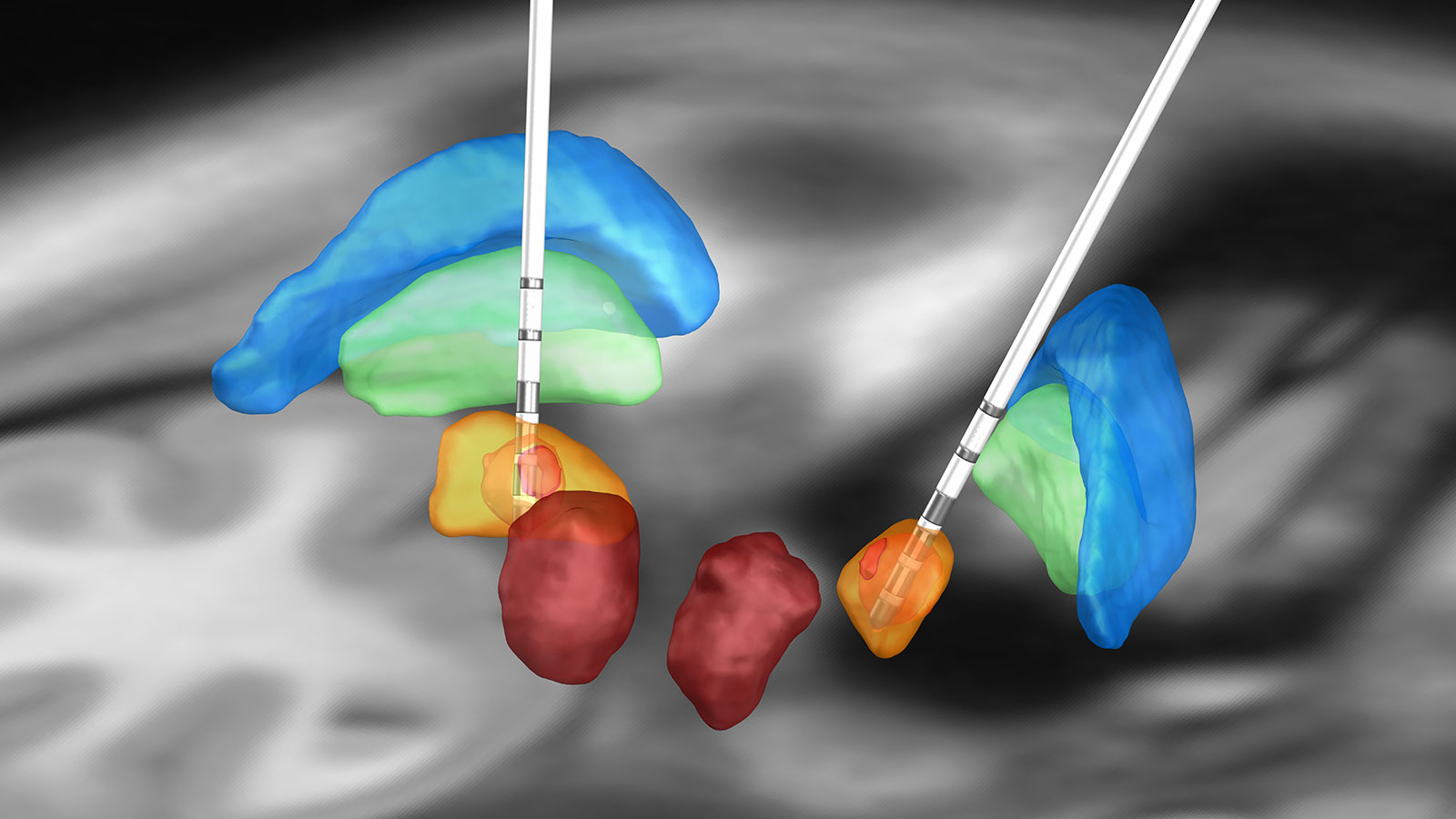
What can a patient with Parkinson's expect from deep brain stimulation?
Deep brain stimulation (DBS) for Parkinson's disease can neither cure the disease nor stop the disease process. It only alleviates the motor symptoms of Parkinson's such as akinesia, tremor and rigor and reduces drug-induced dyskinesias, i.e. disturbances in the course of movement. The effect of DBS in terms of symptom relief is not significantly greater than the effect of dopaminergic drug therapy, but it has a more consistent effect throughout the day. Disruptive dyskinesias occur much less frequently. In addition, when stimulating in the area of the nucleus subthalamicus, medication can be reduced by about half. Overall, stimulation significantly improves patients' quality of life – a fact that has been demonstrated in several high-quality studies.
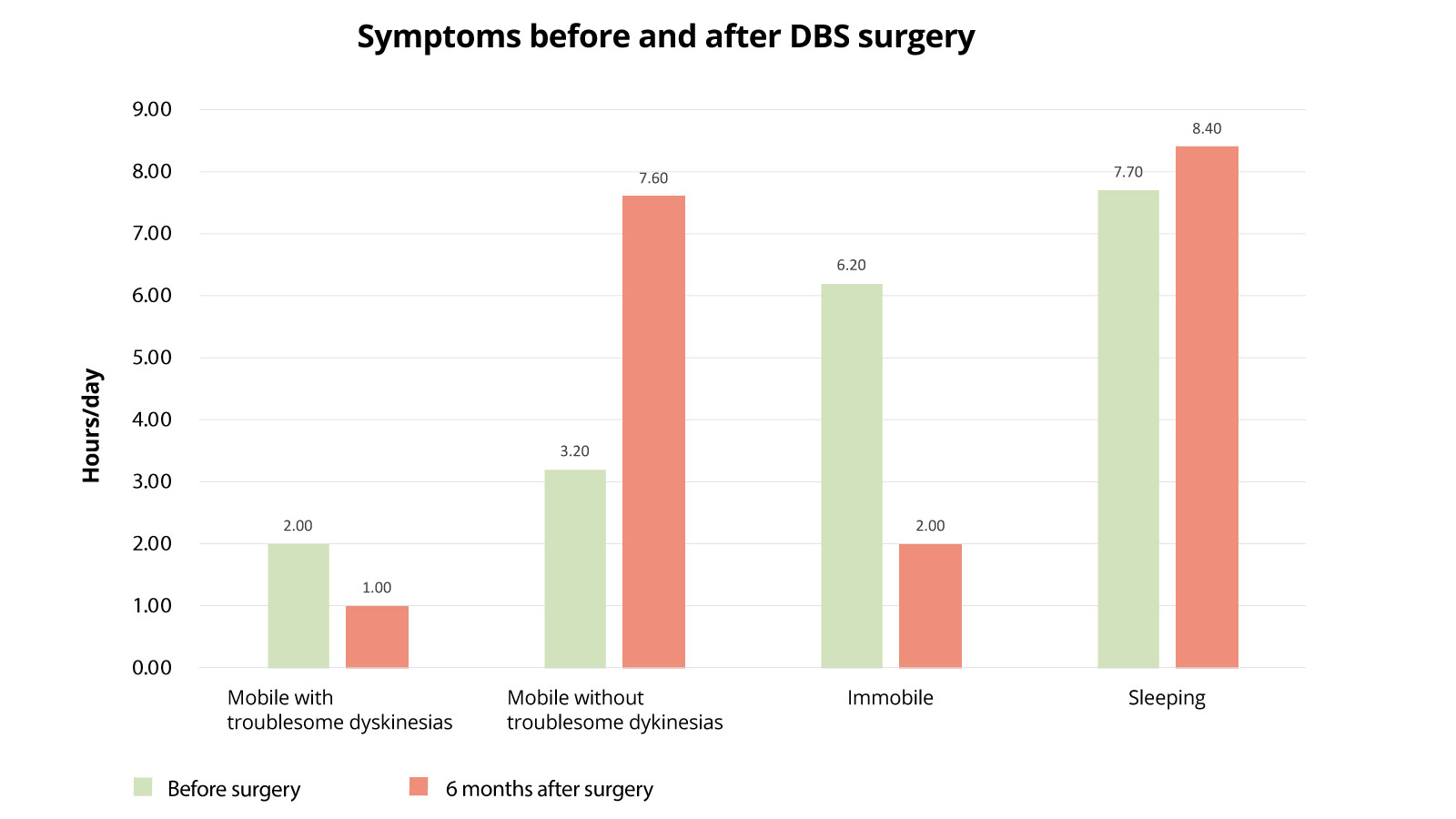
Symptoms before and after DBS surgery
The following diagram shows how the health status of Parkinson's patients changed after DBS surgery. The active times with movement disorders, the active times without movement disorders, times of total immobility and sleep times are examined. The state before surgery is compared with the state 6 months after surgery. What is most striking here is that patients spend much less time in an immobile state postoperatively (6.2 hours before surgery versus 2 hours after surgery), so they can be significantly more active. These activity times are also much less burdened by movement restrictions than before surgery (activity without burdening movement disturbances was 3.2 hours before surgery and 7.6 hours postoperatively). Here, too, a significant improvement is demonstrable.
What are the side effects of deep brain stimulation?
As a general rule, the same side effects apply as with all stereotactic, functional DBS procedures. Specific risks with GPi or STN stimulation for the treatment of Parkinson's disease can be transient perceptions of "light flashes" when stimulating the optic tract, as well as muscular twitching, speech disorders (dysarthria) or sensory disturbances. Usually, these stimulation-induced side effects are reversible. In large studies, a deterioration in cognition after DBS implantation has been reported in about 10–15% of patients.
Are all Parkinson's patients eligible for deep brain stimulation?
Not all patients are candidates for surgical therapy. Suitable candidates meet the following criteria:
- The patient shows severe motor impairments (e.g. dyskinesia) or a strong tremor despite good drug treatment.
- The patient's drug therapy is subject to strong fluctuations and sudden losses of effectiveness in the course of the day (wearing-off, fluctuations in effectiveness).
- The patient has no severe cognitive problems (dementia), behavioral problems, or depression.
Tremor, fluctuations in the effectivenss, and relief of dyskinesias respond best to surgical therapy. Autonomic symptoms (blood pressure fluctuations, micturition disorders, salivation, etc.) and cognitive symptoms (Parkinson's dementia) cannot be improved at this stage. However, several studies are currently underway. Deep brain stimulation of the pedunculopontine nucleus for freezing of gait has shown mixed results *.. Decisions must be made on a case-by-case basis. The indication for DBS is generally made jointly by neurologists and neurosurgeons after time-consuming examinations (neurological examinations, neuropsychological tests, psychiatric evaluation, surgical evaluation) to ensure that only patients with a high chance of success in improving their symptoms are selected for the procedure.
Why you should seek treatment at Inselspital
We are the only center in Switzerland to routinely perform the DBS surgery for Parkinson's disease under general anesthesia.
The advantages are
- very precise implantation of the electrode
- no stressful awake operation
- shorter operation time
- gentler procedure for our patients
- no stopping of Parkinson's medication before surgery
The change from awake surgery to surgery under general anesthesia is based on the experience of major international centers and scientifically published results *. We have been recommending and practicing the implantation of electrodes and pacemakers under general anesthesia since 2021. In our experience, the results of surgery under anesthesia are just as good or even better than with awake surgery. However, this requires a high level of expertise and a great deal of experience on the part of the treating physicians. We have also found that all our Parkinson's patients prefer surgery under general anesthesia.
This innovative procedure has been made possible by
- the improvement in modern imaging, which allows a precise and accurate implantation of the electrode even under general anesthesia
- the technological advancement of the implanted systems (segmented electrodes).
As with awake surgery, electrophysiological signals from the brain are derived during the operation under general anesthesia and intraoperative test stimulation is also carried out to test for side effects. In this way, the placement of the electrode can be checked with millimeter precision. If brain signals are not derived and/or side effects occur rapidly, the electrode is repositioned and tested again.
If medically indicated, the procedure can also be performed as an awake surgery in individual cases. Our treatment team will discuss this with you in detail in advance and also explain the procedures for awake surgery, should this be necessary. In patients with severe dystonia, however, the procedure must always be performed under general anesthesia.
-
Bluett B, Bayram E, Litvan I. The virtual reality of Parkinson’s disease freezing of gait: A systematic review. Parkinsonism & related disorders. 2019
-
Cif L, Hariz M. Seventy Years with the Globus Pallidus: Pallidal Surgery for Movement Disorders Between 1947 and 2017. Mov Disord. 2017;32:972-982.
-
Deuschl G, Schade-Brittinger C, Krack P et al. A Randomized Trial of Deep-Brain Stimulation for Parkinson's Disease. New England Journal of Medicine. 2006;355:896-908.
-
Odekerken VJJ, van Laar T, Staal MJ et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. The Lancet Neurology. 2013;12:37-44.
-
Nowacki A, Galati S, Ai-Schlaeppi J, Bassetti C, Kaelin A, Pollo C. Pedunculopontine nucleus: an integrative view with implications on deep brain stimulation. Neurobiology of disease. 2019;128:75-85.
-
Holewijn RA, Verbaan D, van den Munckhof PM, Bot M, Geurtsen GJ, Dijk JM, Odekerken VJ, Beudel M, de Bie RMA, Schuurman PR. General Anesthesia vs Local Anesthesia in Microelectrode Recording-Guided Deep-Brain Stimulation for Parkinson Disease: The GALAXY Randomized Clinical Trial. JAMA Neurol. 2021 Oct 1;78(10):1212-1219.