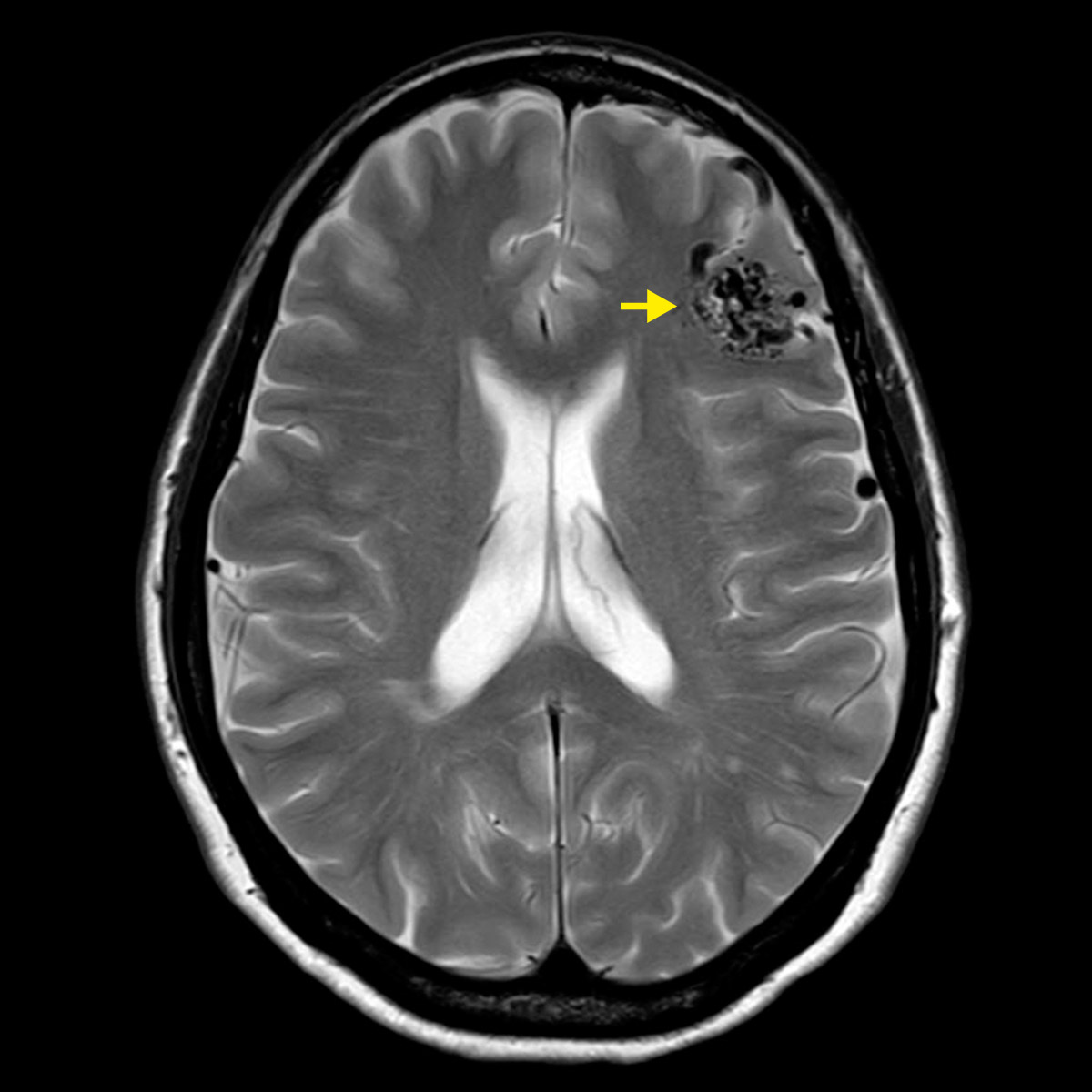
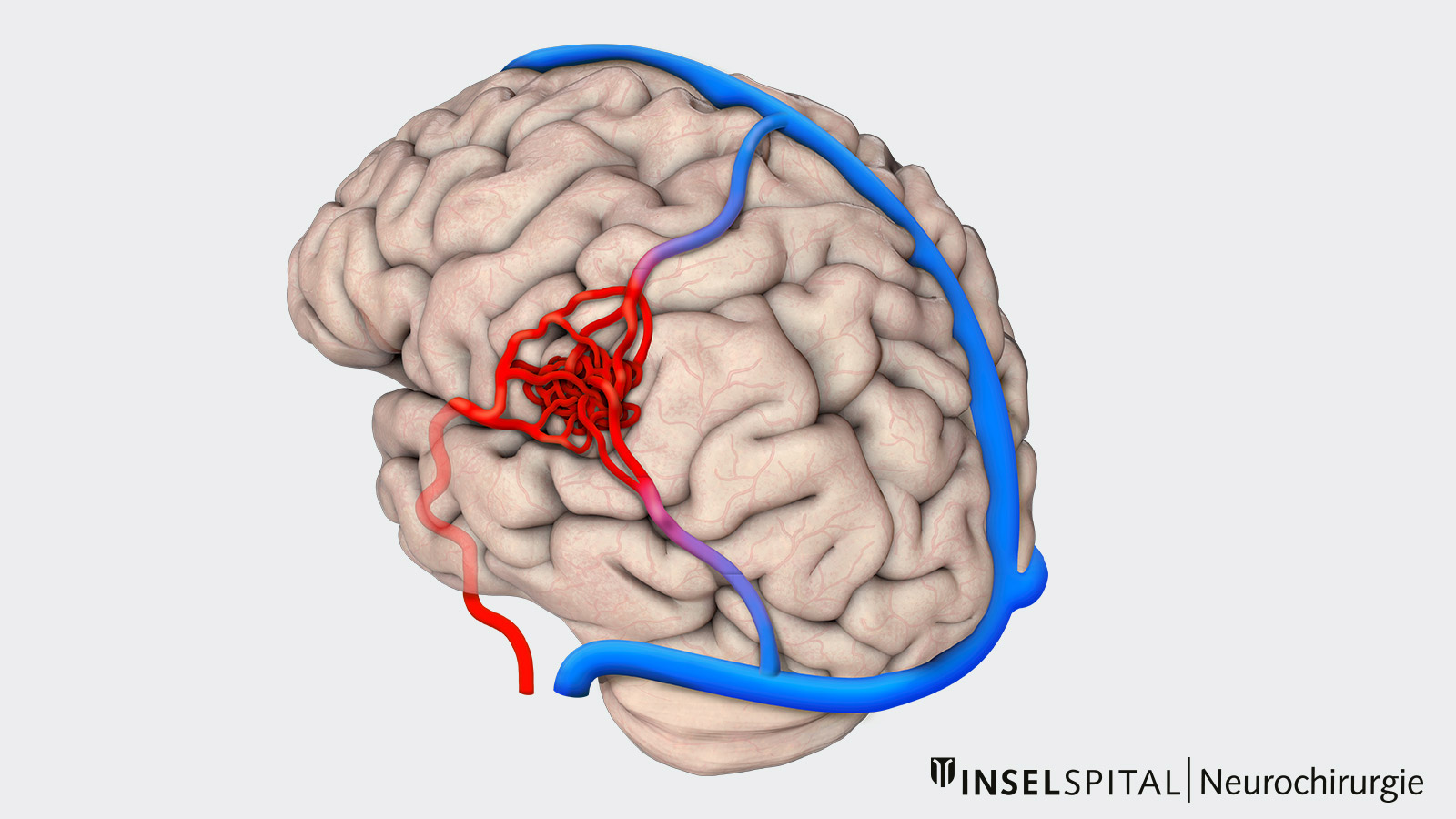
An arteriovenous malformation (AVM) is a malformation of the blood vessels. It consists of a network of vessels in which blood-supplying cerebral arteries and blood-draining cerebral veins are directly connected. The center of the knotted blood vessels is called the nidus. No small capillary vessels are interposed between the arteries and veins in the nidus, allowing blood to flow unrestrained through the vessels at high flow and pressure. In the event of a rupture, severe intracerebral hemorrhage with damage to the surrounding brain tissue can occur.
How common is an AVM and who is affected?
AVMs are very rare and usually congenital. Statistically, an AVM is diagnosed in 1.34 out of 100,000 people per year. In Switzerland, this is approximately 120 people a year. The estimated incidence is 0.14% of the population*, *. Patients suffering from Osler-Rendu-Weber syndrome have an increased risk of AVM (about 5–13% of those affected)*.
What are the symptoms?
AVMs are most commonly diagnosed in young adulthood. They are the main cause of intracranial hemorrhage at this age *. The most common symptoms are usually *, *, *:
- cerebral hemorrhages with stroke-like symptoms (40‒70%).
- epileptic seizures (10‒30%)
- neurological deficits
- headaches
What is the risk of bleeding?
The risk of bleeding from an AVM depends on several factors. Generally, AVMs diagnosed due to a brain hemorrhage have a higher risk of bleeding than AVMs diagnosed due to other symptoms or incidentally.
A meta-analysis of 18 studies involving 8 418 AVM patients showed a mean annual risk of bleeding of 2.2% for unruptured and 4.3% for ruptured AVMs.* In the first year after diagnosis, the risk of bleeding is higher: 2.9% for unruptured and 6‒33% for ruptured AVMs.*, *
Apart from previous hemorrhage, other potential factors for increased risk of rupture are:
- deep venous drainage
- venous ectasia
- intracranial aneurysm, i.e. ballooning of a blood vessel in the brain*
According to the results of the largest cohort studies, the mortality of a ruptured AVM with subsequent hemorrhage is about 25%, and the probability of permanent damage is about 50‒60%.*, *, *, *, *, *
Why you should seek treatment at Inselspital
In view of the complexity of the clinical picture and the various treatment options, an interdisciplinary assessment is essential. Images of patients with an AVM are discussed and evaluated in detail at our weekly conference by experts from the fields of neurosurgery, neuroradiology and radiotherapy.
All decisions about potential AVM treatment weigh the risk of spontaneous bleeding from the untreated AVM against the risk of treatment or the combination of multiple treatments. The latter aims to still have a lower complication risk in the combination of multiple procedures compared to treatment with a single procedure alone.
How are AVMs classified?
There are several classification scales for grading AVMs that help the doctor to better assess the risk of treatment for an AVM. Confirmed in several cohort studies are
- the Spetzler-Martin scale (5 grades)
- the simplified Spetzler-Ponce scale (3 grades A, B and C) *, *
- the Supplemented-Spetzler-Martin scale *
The Spetzler-Martin scale takes into account the size of an AVM, its localization near sensitive brain areas and the presence of deep venous blood drainage
How is an AVM treated?
For low-grade AVMs (Spetzler-Martin grade I–III), we usually recommend treatment because the risk of bleeding, at 2–2.5% per year, is much higher overall than the risk of complications from treatment.
Higher-grade AVMs (Spetzler-Martin grade IV–V) are usually checked regularly by a team of specialists because of the high risk of complications from treatment. If higher-grade AVMs bleed repeatedly or the neurological deficits increase sharply, treatment is considered, which then often consists of a combination of different procedures (embolisation, radiosurgery, surgery).
Embolization
Embolization is the occlusion of vessels via a catheter. Usually, the catheter is first guided into the brain via the inguinal artery and then advanced into an AVM vessel. X-rays are used to check the position in the AVM and the vessel is taped shut. This procedure can completely close up to 30% of AVMs. It is also often used for larger AVMs (Spetzler-Martin grade III) as a preparation for surgery to reduce the risk of bleeding during surgery. Endovascular surgery is also often used to close aneurysms that can form near AVMs.
Surgery
Surgery to completely remove the lesion is the way to completely eliminate the risk of bleeding. In selected cases, artificial occlusion (embolization) of the feeding AVM vessels can be considered before the actual surgery. This can reduce the risk of bleeding during the operation. During the operation, the arteries of the AVM (feeders) are closed off one by one until the entire nidus is cut off from the blood supply. Often, normal brain vessels also pass close to the AVM and have to be distinguished from the feeders. Only when all feeders are closed can the vein be closed and the AVM removed from the brain.
Radiosurgery
Stereotactic radiosurgery may be considered in cases where the AVM is deep in the brain or affects important brain functions. It is mainly used to treat small AVMs (< 3 cm). Larger AVMs have been radiated, alone or in combination with surgery or embolization. Radiosurgery leads to a thickening of the inner vessel wall, called intimal hyperplasia, which leads to a slow vessel occlusion over 2–3 years. During this time, there is still a risk of AVM rupture. In addition, depending on the total dose of radiation administered, complications such as radiation necrosis may occur.*
Results of the treatment of AVM
A meta-analysis and systematic review of all treatments for AVMs showed the following figures for successful closure in AVM patients *:
| Complete closure | Complication rate | |||
|---|---|---|---|---|
| SM I–II | SM III | SM I–II | SM III | |
| Embolization | approx. 45% | approx. 45% | 6–24% | 6–24% |
| Radiosurgery (5y) | approx. 70% | approx. 70% | 6% | na |
| Microsurgery | > 98% | > 98% | 5–10% | 15–20% |
-
Choi J, Mohr J. Brain arteriovenous malformations in adults. The Lancet Neurology. 2005;4(5):299-308.
-
Laakso A, Dashti R, Seppänen J, Juvela S, Väärt K, Niemelä M et al. Long-term Excess Mortality in 623 Patients with Brain Arteriovenous Malformations. Neurosurgery. 2008;63:244-255.
-
Willemse R, Mager J, Westermann C, Overtoom T, Mauser H, Wolbers J. Bleeding risk of cerebral vascular malformations in hereditary hemorrhagic telangiectasia. Journal of Neurosurgery. 2000;92(5):779-784.
-
Al-Shahi R. A systematic review of the frequency and prognosis of arteriovenous malformations of the brain in adults. Brain. 2001;124(10):1900-1926.
-
Derdeyn C, Zipfel G, Albuquerque F, Cooke D, Feldmann E, Sheehan J et al. Management of Brain Arteriovenous Malformations: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2017;48(8).
-
Fullerton H, Achrol A, Johnston S, McCulloch C, Higashida R, Lawton M et al. Long-Term Hemorrhage Risk in Children Versus Adults With Brain Arteriovenous Malformations. Stroke. 2005;36(10):2099-2104.
-
Goldberg J, Raabe A, Bervini D. Natural history of brain arteriovenous malformations: systematic review. Journal of neurosurgical sciences. 2018;62(4):437‐443.
-
Graf C, Perret G, Torner J. Bleeding from cerebral arteriovenous malformations as part of their natural history. Journal of Neurosurgery. 1983;58(3):331-337.
-
Mast H, Young W, Koennecke H, Sciacca R, Osipov A, Pile-Spellman J et al. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. The Lancet. 1997;350(9084):1065-1068.
-
Gross B, Du R. Natural history of cerebral arteriovenous malformations: a meta-analysis. Journal of Neurosurgery. 2013;118(2):437-443.
-
Brown R, Wiebers D, Forbes G, O'Fallon W, Piepgras D, Marsh W et al. The natural history of unruptured intracranial arteriovenous malformations. Journal of Neurosurgery. 1988;68(3):352-357.
-
Crawford P, West C, Chadwick D, Shaw M. Arteriovenous malformations of the brain: natural history in unoperated patients. Journal of Neurology, Neurosurgery & Psychiatry. 1986;49(1):1-10.
-
da Costa L, Wallace M, ter Brugge K, O'Kelly C, Willinsky R, Tymianski M. The Natural History and Predictive Features of Hemorrhage From Brain Arteriovenous Malformations. Stroke. 2009;40(1):100-105.
-
McAllister J, Eskandari R, Limbrick D: Youmans & Winn neurological surgery. 2017
-
Ondra S, Troupp H, George E, Schwab K. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. Journal of Neurosurgery. 1990;73(3):387-391.
-
van Beijnum J, van der Worp H, Buis D, Salman R, Kappelle L, Rinkel G et al. Treatment of Brain Arteriovenous Malformations. JAMA. 2011;306(18):2011.
-
Spetzler R, Martin N. A proposed grading system for arteriovenous malformations. Journal of Neurosurgery. 1986;65(4):476-483.
-
Spetzler R, Ponce F. A 3-tier classification of cerebral arteriovenous malformations. Journal of Neurosurgery. 2011;114(3):842-849.
-
Lawton M, Kim H, McCulloch C, Mikhak B, Young W. A Supplementary Grading Scale for Selecting Patients With Brain Arteriovenous Malformations for Surgery. Neurosurgery. 2010;66(4):702-713.
-
Vlaskou Badra E, Ermiş E, Mordasini P, Herrmann E. Radiosurgery and radiotherapy for arteriovenous malformations: outcome predictors and review of the literature. J Neurosurg Sci. 2018;62(4):490‐504.