Glioblastoma is the most common malignant brain tumor in adults. It develops from the glial cells of the brain and mainly occurs in older people. The standard treatment consists of a combination of surgery, radiotherapy and chemotherapy, known as the Stupp regimen. The prognosis for glioblastoma is unfavourable, so the main focus is on improving the patient's quality of life and maximizing survival time.
Inselspital, Department of Neurosurgery – our facts and figures
- Excellence: highly specialized neurosurgeons and specially trained oncology nurses (Advanced Practice Nurses or APNs for short).
- Expertise: 417 tumor surgeries (biopsies and resections) in 2024, of which 359 were gliomas
- Interdisciplinary team: specialists from 7 specialties meet in our weekly tumor board – neurosurgery, neurology, neuroradiology, oncology, nuclear medicine, radiation oncology, pathology
- Developed and researched at Inselspital: innovative neuromonitoring and navigation techniques for surgical safety and avoidance of deficits
- Deficit rate among the lowest worldwide: documented and published rate of 3–5% for permanent surgery-related deficits in motor eloquent risk tumors is among the lowest worldwide!
- Resection rate among the highest worldwide: The rate of > 90% complete resection for gliomas is among the highest worldwide!
- State-of-the-art technological equipment with intraoperative imaging, fluorescence techniques, laser thermotherapy and more.
- Complementary treatment concept: our OPTIMISST protocol (OPTIMISST stands for Optimized Standard and Supportive Therapy).
- Certification: Certified Brain Tumor Center since 2016, guaranteeing a high quality standard in oncological treatment.
What is a glioblastoma?
Glioblastomas (also known as glioblastoma multiforme due to the different appearance of the tumor parts) are the most common malignant brain tumors and account for 55% of primary brain tumors that develop in the brain and are not metastases.
Glioblastomas grow along the fiber tracts in the brain and thus spread locally, regionally and supraregionally. Glioblastomas do not usually cause metastases outside the nervous system.
According to the World Health Organization (WHO), a glioblastoma is classified as WHO grade 4, which is the highest level of malignancy. This classification is based on characteristics such as
- high cell division rate
- necrosis (tissue death)
- rapid growth of new blood vessels (vascular proliferation)
In addition, molecular analysis can reveal specific genetic alterations such as mutations in the IDH1/IDH2 gene or methylation of the MGMT promoter, which have prognostic and therapeutic significance.
Rare variants of glioblastoma are gliosarcoma, giant cell glioblastoma and epithelioid glioblastoma.
- Molecular subgroups
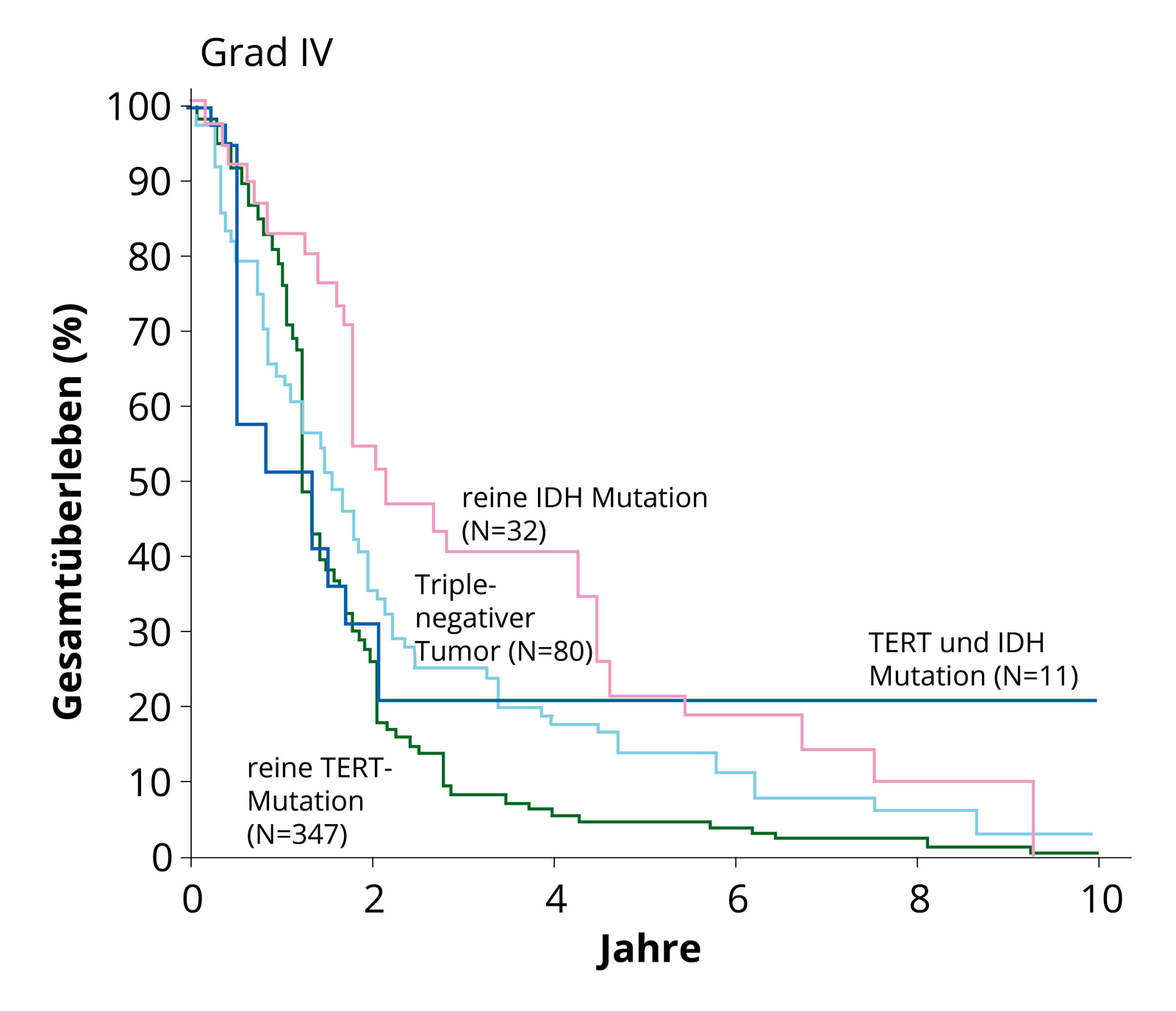
Survival curves of the molecular subgroups. It is clearly evident that glioblastomas without IDH mutation (IDH wild type) with TERT mutation show poorer survival (green line) than tumors with existing IDH mutation. Quelle: Eckel-Passow et al, N Engl J Med 2015; 372:2499–508. Frequently found in primary glioblastomas are
- Mutations in the EGF receptor
- Mutations in the PTEN gene
- Mutations in the TERT gene
The EGF receptor receives signals from a cell's environment that mediate cell growth and division and is often overactive in primary glioblastomas.
The product of the PTEN gene is a tumor suppressor that plays an important role in the regulation of cell growth.
The product of the TERT gene is an enzyme that prevents the progressive shortening of chromosome ends during rapid cell division. This delays the biological ageing of a cell, known as cellular senescence.
Secondary glioblastomas are characterized by a mutation in the IDHgene and often also by a mutation in the p53 gene, a tumor suppressor.
Large-scale genetic studies such as the analyses of The Cancer Genome Atlas (TCGA) project have provided further important insights into the genetic fingerprint of different subtypes of glioblastoma *.
The various genetic subgroups of glioblastomas differ in their behavior.
- Methylation
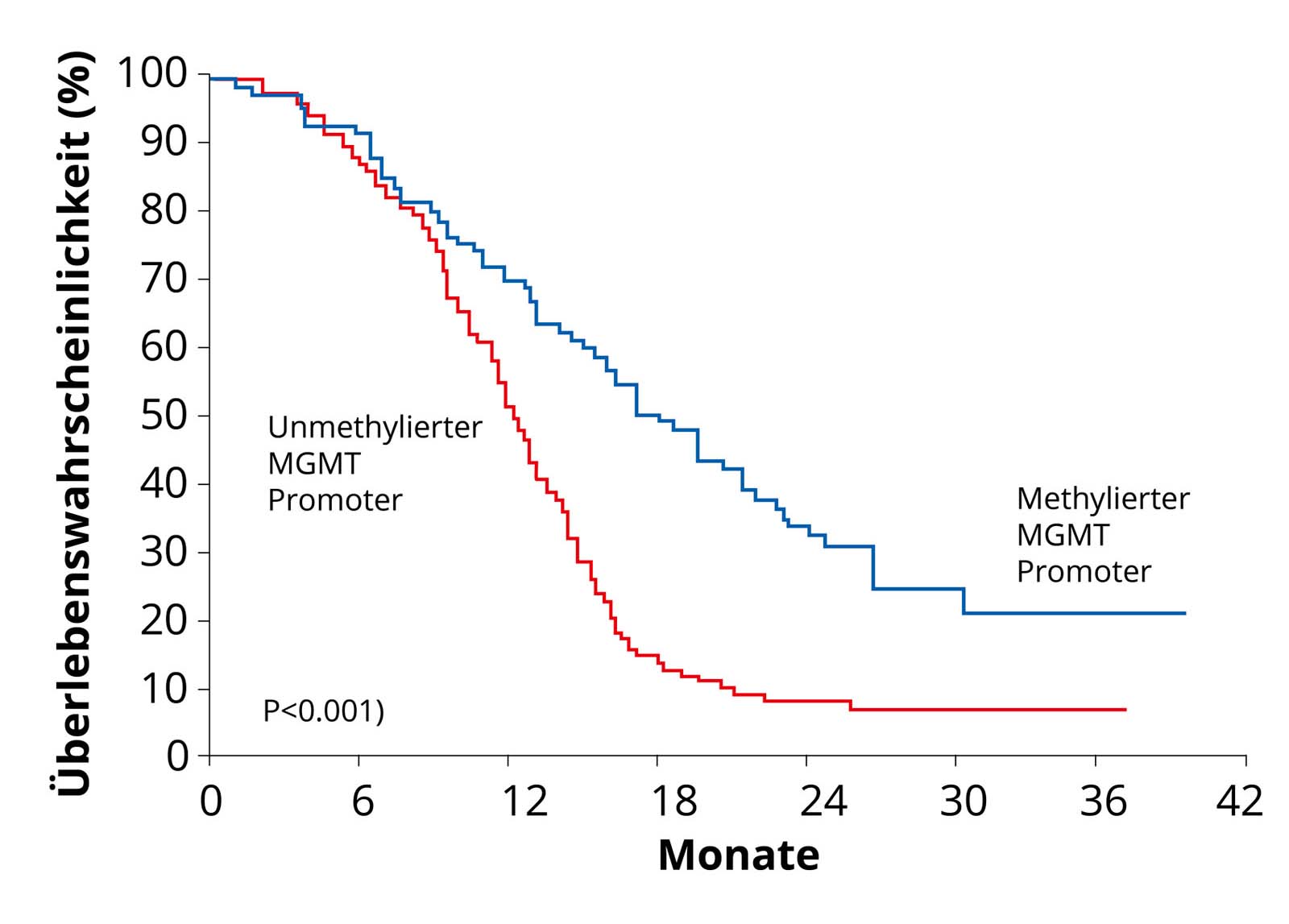
Glioblastomas with methylation of the MGMT promoter. As these tumors respond better to chemotherapy, these patients have a better survival curve (blue curve) than patients with non-methylated MGMT promoter (red curve) Quelle: Hegi et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N Engl J Med 2005; 352: 997-1003. An important genetic modification of glioblastoma cells from a therapeutic perspective is the so-called DNA methylation of the MGMT promoter. DNA methylation is the transfer of methyl groups to specific sites on the genetic material (DNA) of a cell. Methylation therefore modifies the DNA of a glioblastoma cell. This modification shuts down the production of the DNA repair protein MGMT. If a tumor can no longer repair its DNA, it is killed more quickly by chemotherapy. Approximately half of all glioblastomas have this genetic modification. These glioblastoma patients respond better to chemotherapy with Temodal – methylation is therefore an advantage.
In the future, methylome-based tumor classification will become increasingly important *. This involves testing the DNA of the tumor for additional methyl groups. These methyl groups can be acquired and also provide an indication of the cellular origin of the tumor. This method also promises a high degree of standardization. The digital nature of the methylation data also allows easier exchange between research groups and better classification of unclear cases.
How common is glioblastoma and who is affected?
Glioblastoma typically occurs in older adults (50–85 years) with an average age of 64 years *. Although glioblastomas can occur in children, they account for only 2.9% of all brain tumors in the 0–19 age group *.
Overall, these are rare tumors with approx. 3–4 new diagnoses per 100,000 inhabitants per year *. Men are affected 1.5 times more frequently than women.
What are the causes of glioblastoma?
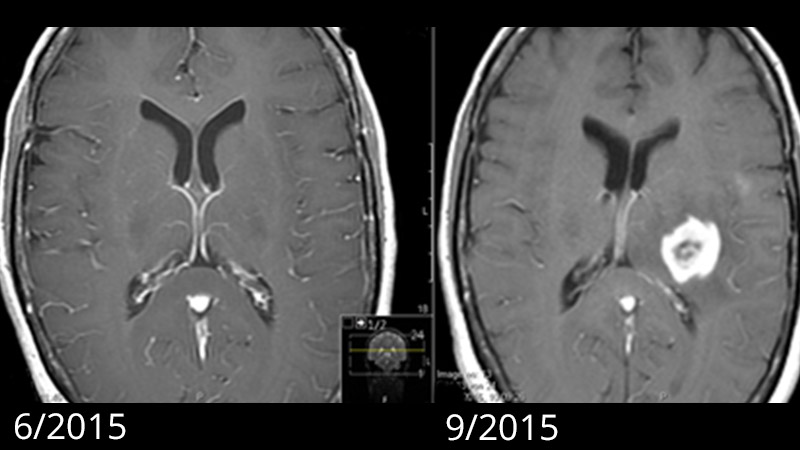
The only confirmed risk factor for the development of a glioblastoma is previous radiation to the head.
A connection with previous head injuries, toxins or diet could not be clearly proven *, *.
Hereditary factors play only a minor role.
Interestingly, patients with allergic diseases (asthma, atopic dermatitis, food allergies, etc.) are said to have a lower risk of developing a glioblastoma *.
New hypotheses assume that the first glioblastoma cell can develop up to 7 years before diagnosis. According to this assumption, the early, critical mutations in tumor development occur 2-7 years before diagnosis. However, it is only when additional mutations occur that the typical rapid growth of glioblastoma is triggered. It is known from patients with previously unremarkable MRI that the glioblastoma visible on imaging has grown rapidly within a few months *, *.
Does radiation from cell phones cause brain tumors?
Large-scale epidemiological studies in humans have so far found no evidence that the use of cell phones leads to an increased risk of developing a brain tumor *, *.
Complex animal studies indicate an increased tumor risk from mobile phone radiation in male rats and mice, but without showing a dose-response relationship and without being able to explain the lack of this effect in female animals.
In humans, the INTERPHONE study found a slightly increased risk of developing glioma with excessive cell phone use *. Another frequently discussed case-control study from Sweden also found a link between the occurrence of gliomas and cell phone use *, *. Based on these two studies, the WHO's International Agency for Research on Cancer (IARC) classifies electromagnetic radio frequency fields as "possibly carcinogenic" *.
In contrast, however, several large-scale epidemiological studies on humans have found no evidence of an increased risk of brain tumors *, *, *, *. This includes the Million Women Study published after the IARC report, which collected prospective data from almost 800,000 participants and found no increased risk *.
Different authorities therefore also classify differently: from "harmless" to "possibly slightly carcinogenic not excluded".
What symptoms does a glioblastoma cause?
The symptoms basically depend on the location of the tumor in the brain. The same applies to all brain tumors:
- Seizures: epileptic activity or hyperexcitability of healthy tissue at the edge of the tumor
- Failures or functional disorders: Impairment of speech, motor function, sensation, vision, calculation, thinking, memory, balance, orientation, mood, behavior, alertness, drive, social behavior, etc. due to pressure of the tumor on neighboring brain structures or ingrowth into the surrounding tissue
- Headaches, nausea, vomiting: an advanced tumor leads to an increase in pressure in the skull
- Nonspecific symptoms: If the tumor grows in functionally silent (non-eloquent) parts of the brain, it may go unnoticed for some time until non-specific symptoms such as changes in personality, fatigue, forgetfulness, disorientation and confusion occur. The symptoms usually last for several weeks to a few months at the time of diagnosis.
Magnetic resonance imaging
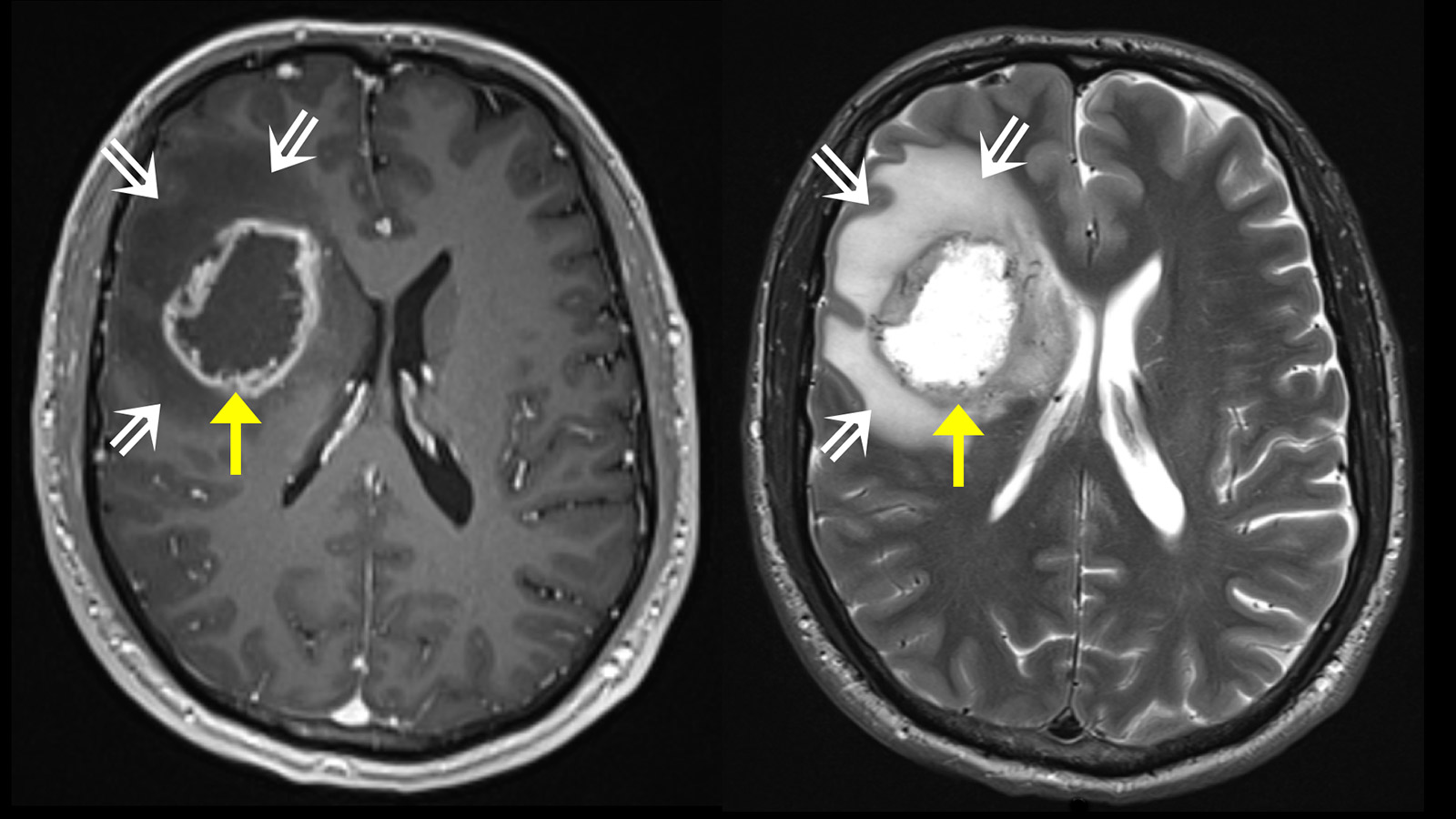
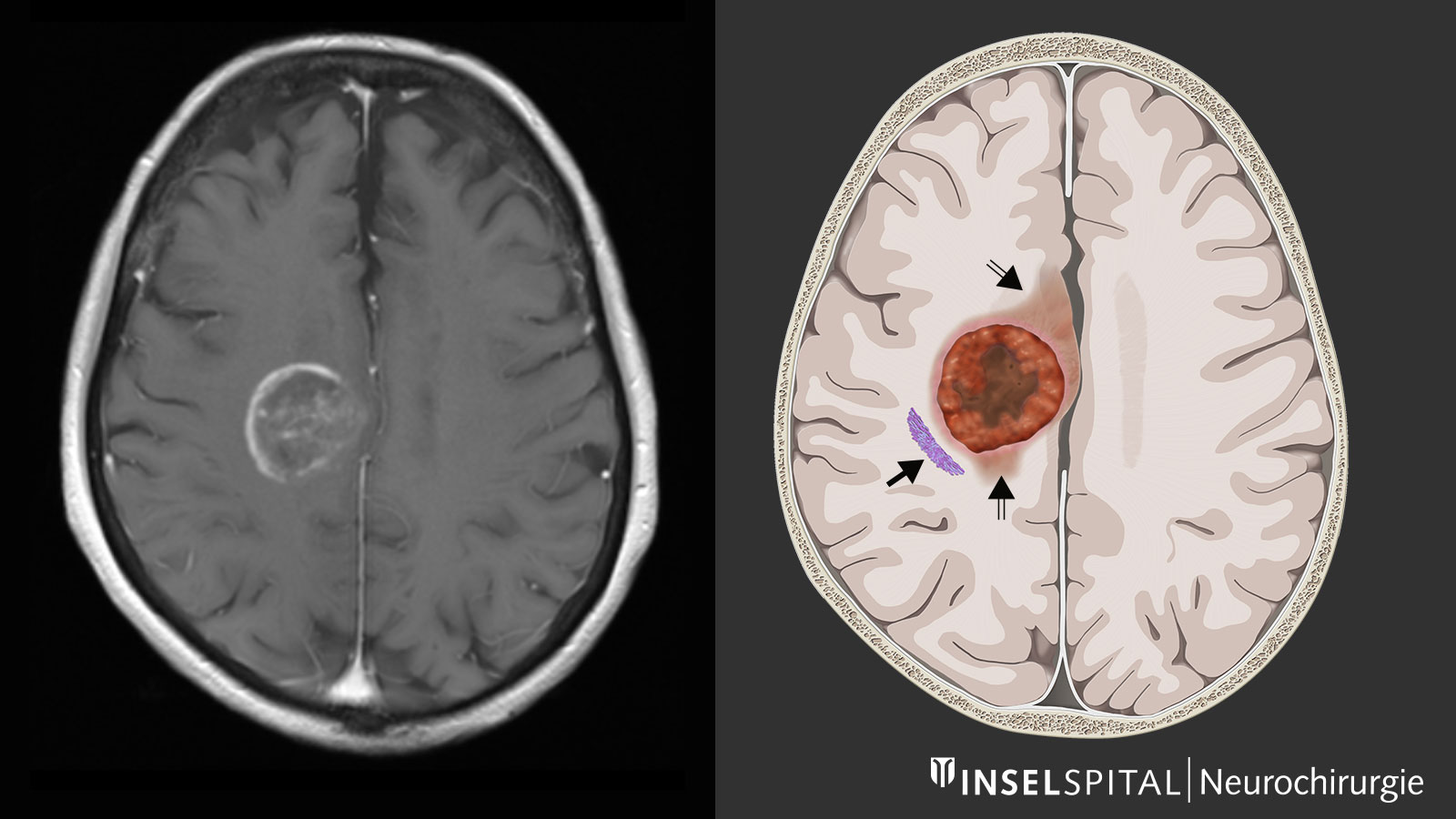
Generally, a magnetic resonance examination of the brain is carried out to clarify the symptoms. Magnetic resonance imaging (MRI) shows the tumor as a heterogeneous area that absorbs contrast medium. Typically, there is a ring-shaped, irregular contrast agent image with a dark area in the center where cells have died (necrosis). In some cases, there is spread along the fiber tracts, which can lead to involvement of various lobes of the brain. Around the tumor, the T2-weighted sequence of the MRI shows a bright area of variable extent. This is a cerebral edema. In contrast to metastases, where this edema is caused by a pure tissue reaction, this zone also contains active tumor cells in glioblastomas.
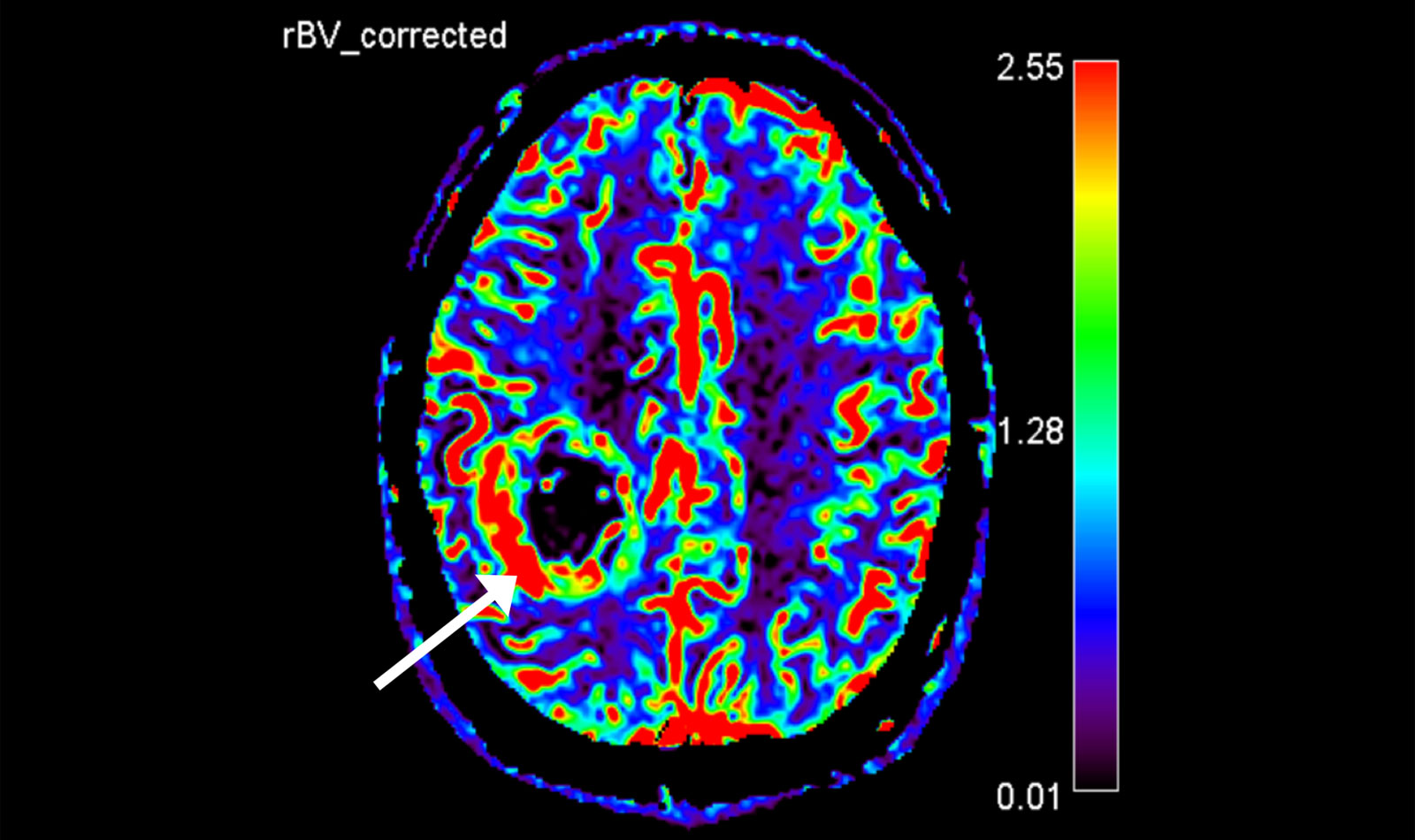
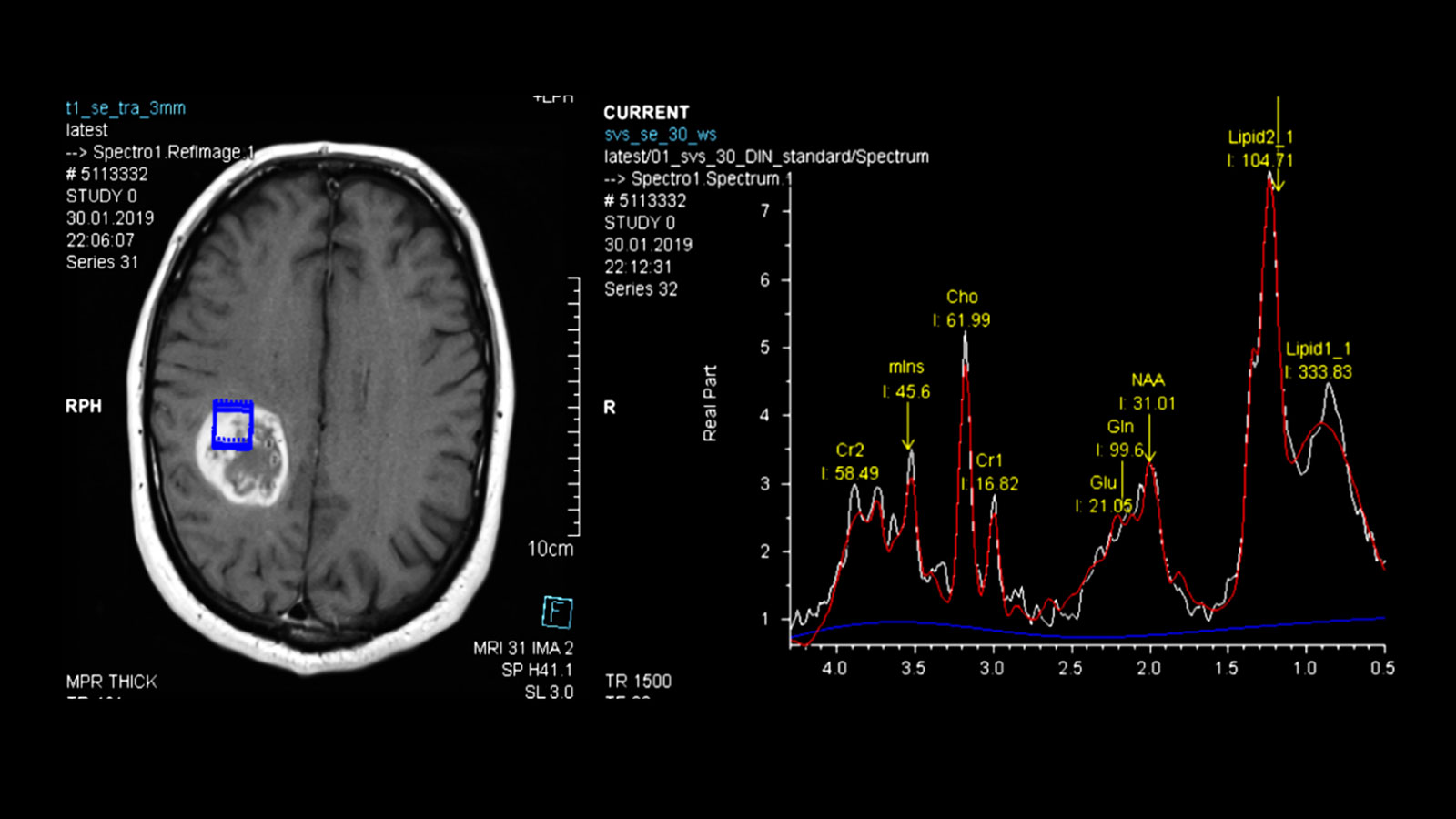
Advanced neuroimaging
Further imaging differentiation can be carried out using advanced neuroimaging.
This includes a method for measuring the local blood flow (perfusion), as the blood volume in the area of the tumor is increased, as well as measuring the local metabolic products (metabolites) using magnetic resonance spectroscopy (MR spectroscopy or MRS), as certain metabolic products are also increased or decreased in gliomas.
The results of the measurements can be particularly helpful in differentiating between glioblastomas and other tumors such as metastases or lymphomas.
Surgery – the first step in therapy
The standard therapy for glioblastomas consists of a combination of microsurgical resection, radiotherapy and chemotherapy.
Surgical resection is now an integral part of the treatment concept *, *, *, *, *, *, *. The more tumor mass that can be removed, the more favourable the further course of the disease *, *, *, *, *, *, *, *, *. Therefore, a complete tumor resection, which is confirmed by an MRI image, is the goal of the operation. Furthermore, the removal of the tumor alleviates the effect that the mass has on the surrounding brain tissue and thus also the symptoms caused by the tumor. The removed tumor tissue can then be examined histologically and molecularly.
However, as glioblastomas infiltrate the brain tissue diffusely and grow along the fibrous pathways of the white matter, tumor cells remain even after a complete resection. These must be destroyed by subsequent radiotherapy and chemotherapy.
Why should radical surgery be aimed for in the case of glioblastoma?
According to current studies, it is assumed that at least 80% of the tumor must be removed or less than 5 cm3 of tumor residue must remain for the patient to have a survival advantage from the operation.
A clear survival advantage can only be achieved by complete removal of the tumor as verified by MRI *, *.
Therefore, the primary goal of surgery should be the complete removal of the brain tumor, ideally including the infiltration zone visible in the MRI image, and this without causing permanent neurological damage.
Surgery with a margin: supramarginal resection
Glioblastomas have tumor cells that extend beyond the margin visible on MRI. This infiltration zone around the tumor has a different biological profile than the tumor core itself * and may have an influence on the prognosis of the therapy *. The infiltration zone surrounding the tumor does not absorb contrast medium and is difficult to delineate radiologically. However, focusing solely on the part of the tumor that is easy to delineate radiologically is not optimal from an oncological point of view.
A further reduction in the tumor burden can be achieved with a more extensive resection up to the functional limits. This is referred to as supratotal or supramarginal resection *, *, *. These supratotal resections are now an integral part of the treatment concept for selected patients.
It is known from tumor surgery that large parts of "functionally silent", so-called non-eloquent regions of the brain can be removed without causing permanent deficits in the patient *. However, supramarginal resection should always be performed simultaneously with neurophysiological monitoring and mapping in order to avoid permanent neurological damage.
How quickly should surgery be performed?
Due to the rapid growth of glioblastomas, surgery should be performed promptly. The more time passes, the more tumor cells migrate into the surrounding area. The tumor expands and becomes larger, the surgical risk increases and the possible radicality of the resection decreases. The operation should therefore ideally be performed within 1–2 weeks of diagnosis.
Our OPTIMISST protocol
There are a number of factors that contribute to a tumor treatment being more successful. We have developed our own treatment concept based on these factors, the so-called OPTIMISST protocol. OPTIMISST stands for "Optimized Standard and Supportive Therapy" and complements conventional therapy for brain tumours. This protocol only exists in this form at our clinic.
Magnetic resonance imaging
For optimal preparation, a special MRI is first performed. This allows the tumor to be assessed as accurately as possible and the operation to be planned precisely. Depending on the localization of the tumor, further examinations may be necessary.
A functional MRI (fMRI) can contribute valuable information on the localization of speech function and the motor center.
Navigated transcranial magnetic stimulation
Navigated transcranial magnetic stimulation (nTMS) is often performed for the precise localization of the centers of motor activity. This is a non-invasive examination that is tolerated by most patients without any problems and is offered at Inselspital as part of a multicenter study. The data collected in this way help the surgeon to plan the surgical approach and to determine the surgical strategy.
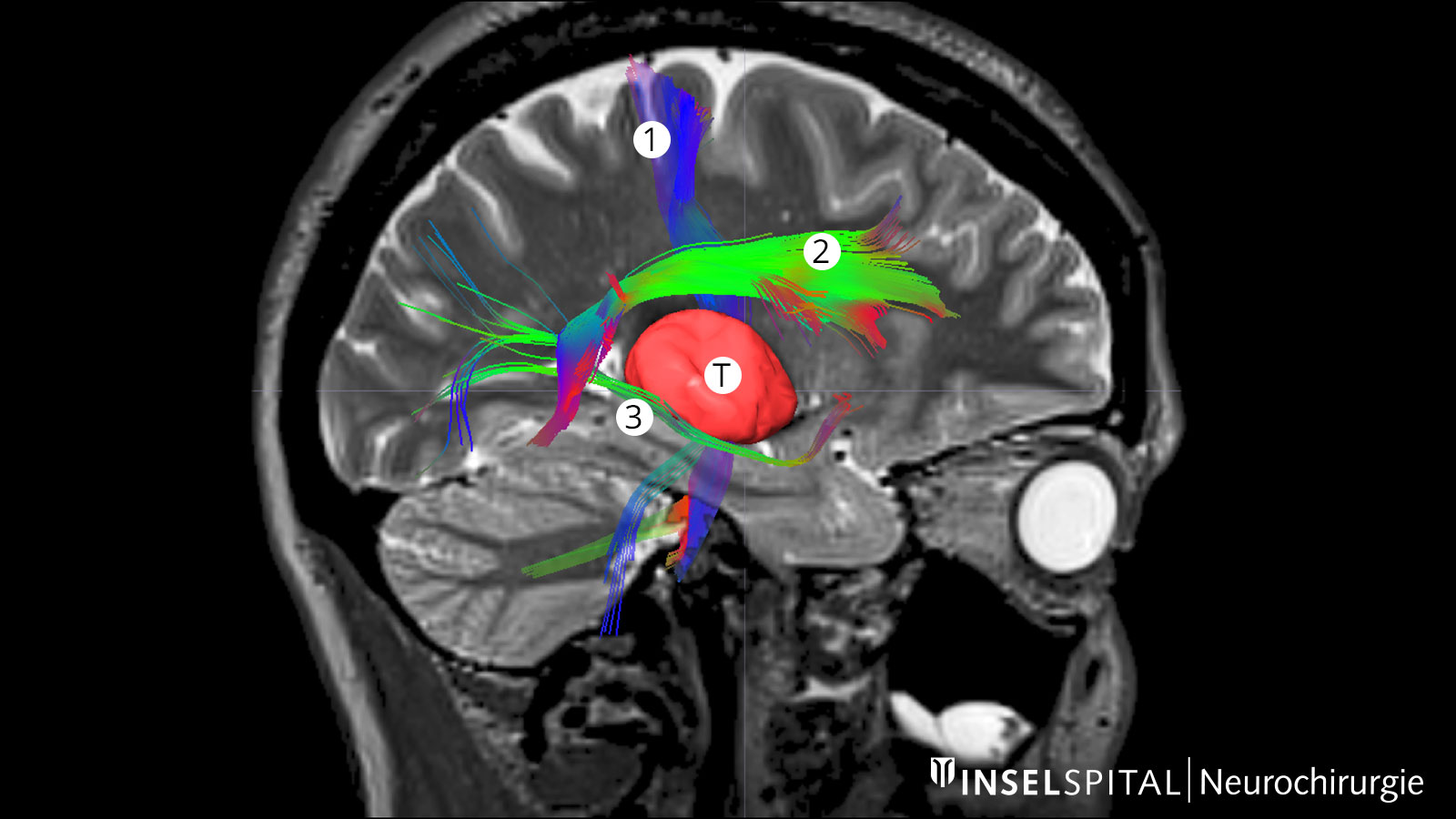
Fiber tracking: visualization of the brain fiber tracts
Tractography or fiber tracking are procedures used to visualize the fiber tracts in the brain. Visualizing these tracts before surgery helps the surgeon to find the optimal and safest path to the tumor past all important fiber tracts.
This is extremely important for the pyramidal tract. It represents the direct connection between the motor center of the brain and the spinal cord and must be spared at all costs during surgery. Other important fiber tracts primarily concern brain functions such as speech and vision.
Should a biopsy be carried out before surgery?
If the tumor can be easily resected, we do not perform a separate biopsy in advance. If resection is not possible due to the location of the tumor in a functionally important center or due to its diffuse extent, a biopsy should at least be performed to examine the tissue and confirm the diagnosis. The risk of complications from a biopsy is low, threatening bleeding only occurs in around 1% of cases.
A more common problem, however, is the incorrect grading of tumors in up to 25% of cases *: As a glioma is often heterogeneously composed of different cell parts, it can happen that cells from a less malignant part are taken in the biopsy and a low-grade glioma is wrongly diagnosed, although it is actually a higher-grade, aggressive glioma.
To reduce the rate of misdiagnosis, we routinely plan biopsies with the help of
- advanced neuroimaging methods such as magnetic resonance spectroscopy (MR spectroscopy or MRS) or
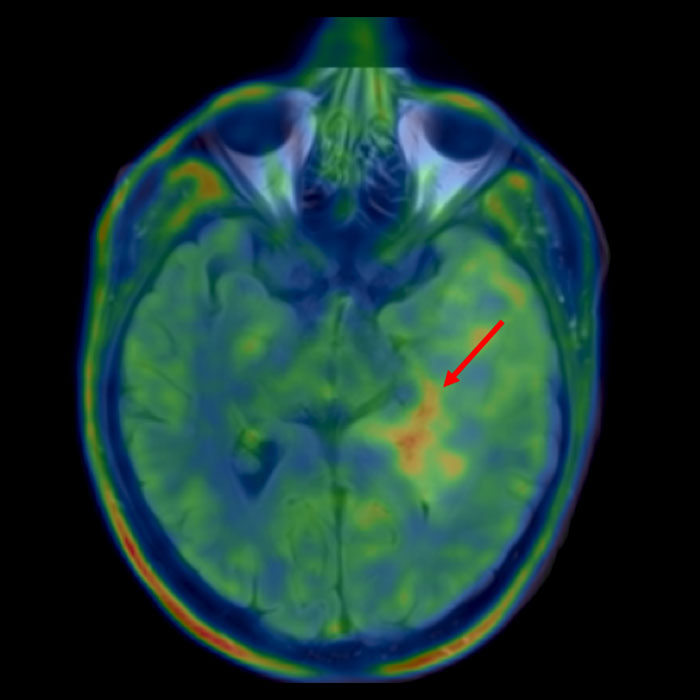
- nuclear medicine methods such as FET-PET. PET (short for positron emission tomography) is an imaging method that allows particularly active tumor areas to be highlighted by administering an "intelligent" molecule, usually fluorethyltyrosine (FET). FET is a labeled amino acid and indicates the increased amino acid metabolism in tumor cells. This can be registered with a camera. Using this method, a hotspot with particularly active tumor cells can often be identified by overlaying the PET data with the MRI data. This hotspot is a good target for a biopsy.
Surgical safety is guaranteed by various innovative techniques. Thanks to precise surgical procedures and extensive intraoperative monitoring, the complication rate of tumor resections is now very low *, *, *, *, *, *, *, *.
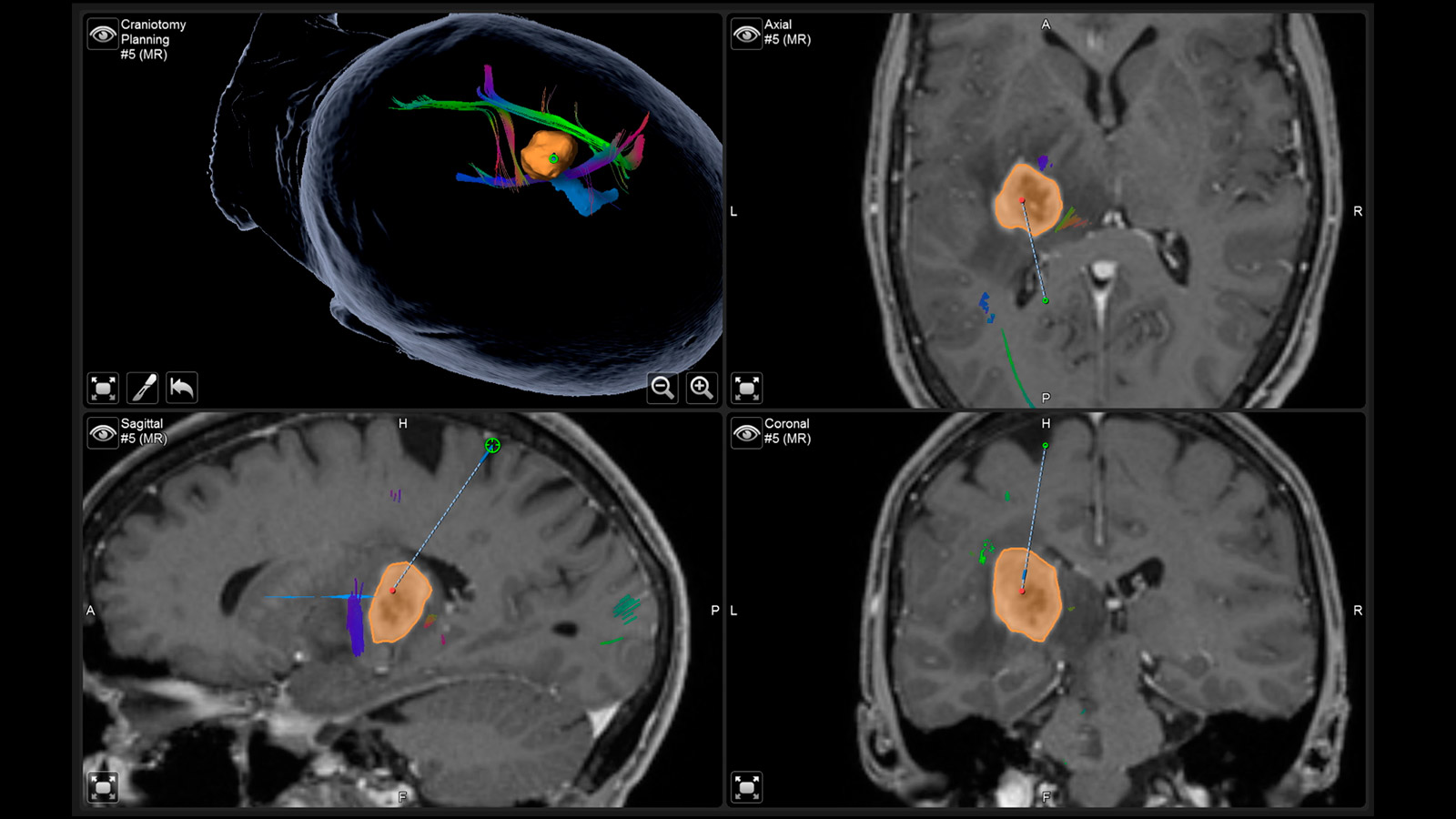
Neuronavigation
Neuronavigation works like a GPS during surgery and shows the neurosurgeon the way, with the MRI performed before surgery providing the individual "map material"
Augmented Reality
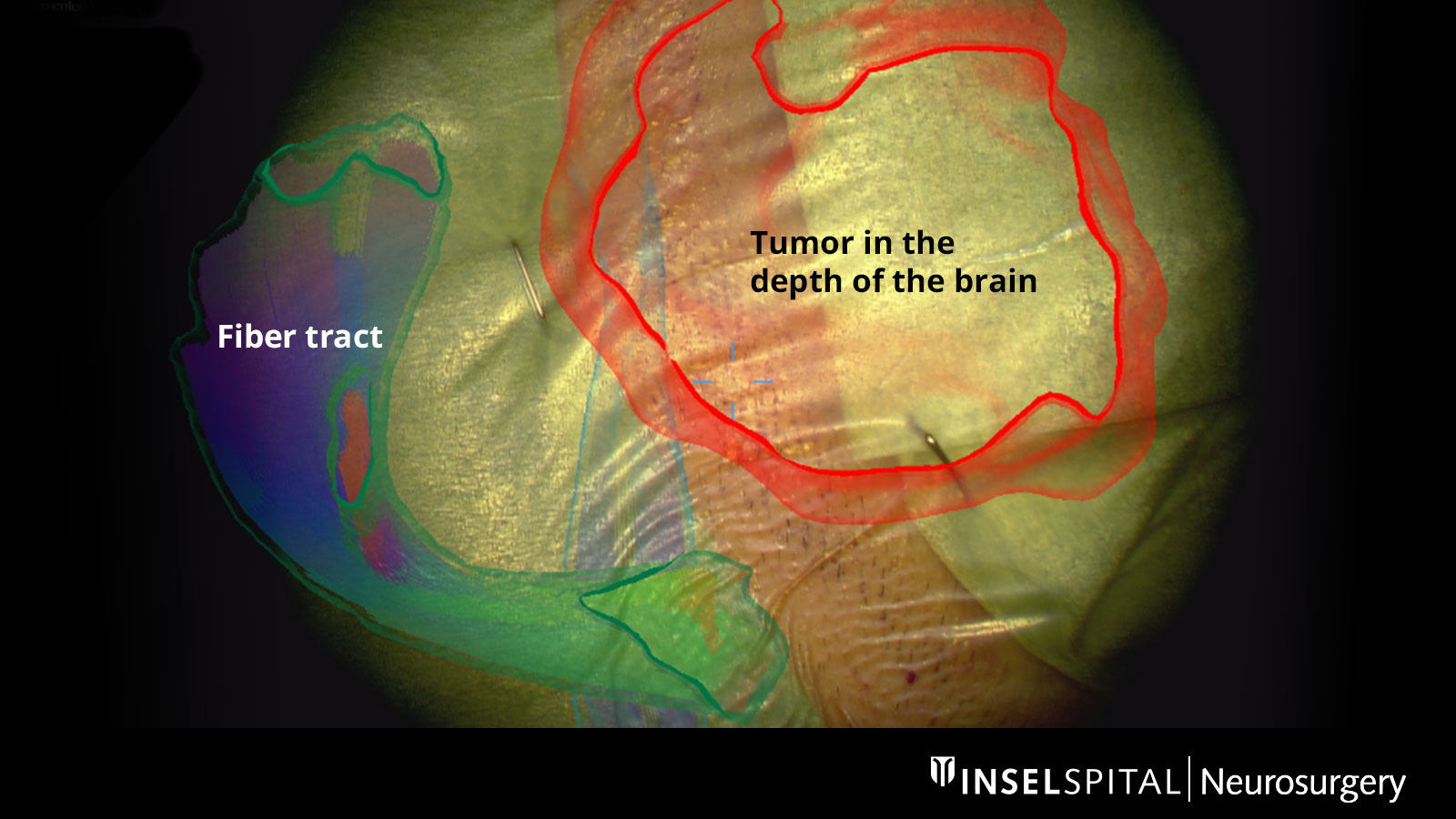
The fiber tracts already drawn in before the operation, as well as the tumor itself and other important areas, can be virtually superimposed in the operating microscope and projected onto the surface of the head. With the help of this augmented reality, the surgeon can orient himself better and plan the access to the tumor as small as possible, but as large as necessary.
Intraoperative neuromonitoring (IOM)
The advantages of radical surgery for glioblastoma only apply if permanent paralysis, speech disorders or other serious damage can be avoided. Otherwise, the survival time decreases again *. Specialized functional monitoring – intraoperative neuromonitoring (IOM) – is intended to prevent surgery-related damage and is in many cases the most important surgical instrument and a specialty of our clinic.
The type of monitoring is determined individually for each patient, taking into account the neighboring brain functions. As a rule, a distance of approx. 3 mm from an important brain area is sufficient for us to remove the tumor without permanent neurological deficits. The following procedures are generally used:
- monitoring: an electrical monitoring frequency to test the functions of the entire brain and nerve conduction pathway
- dynamic mapping: a radar based on continuous microcurrents to determine the distance to the brain center or the brain pathway
- short-term stimulation with "interference current" during awake operations
Monitoring
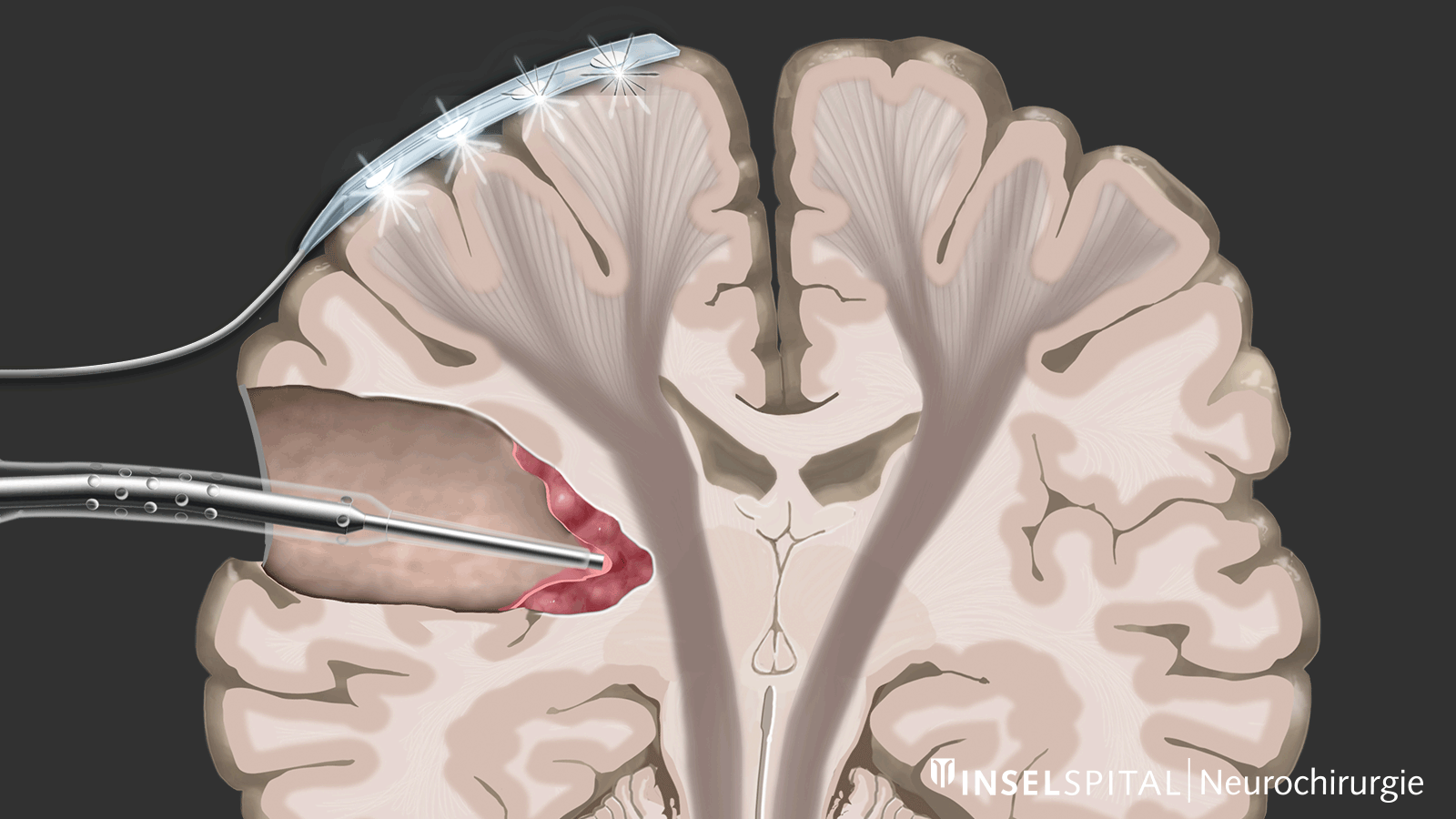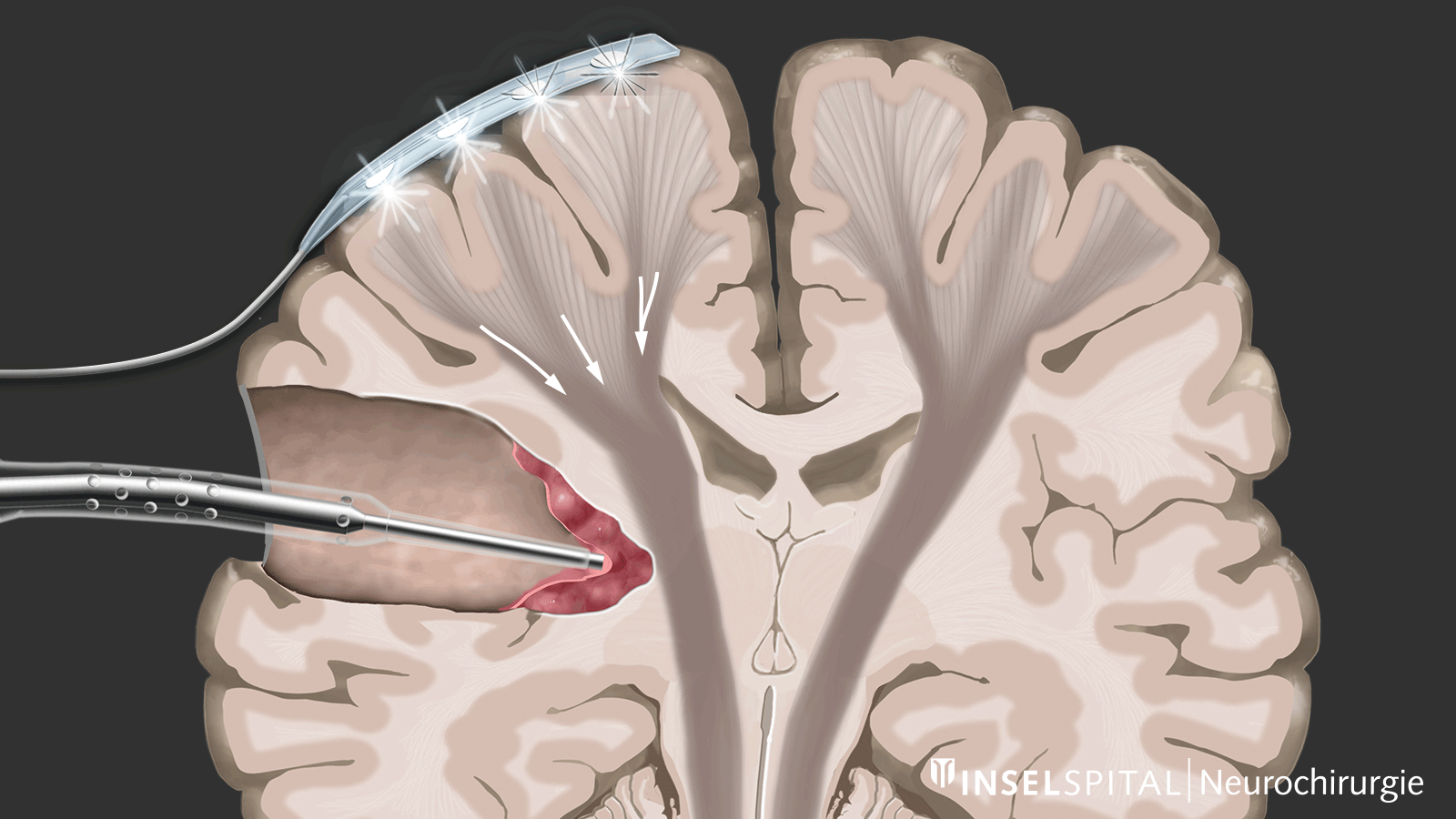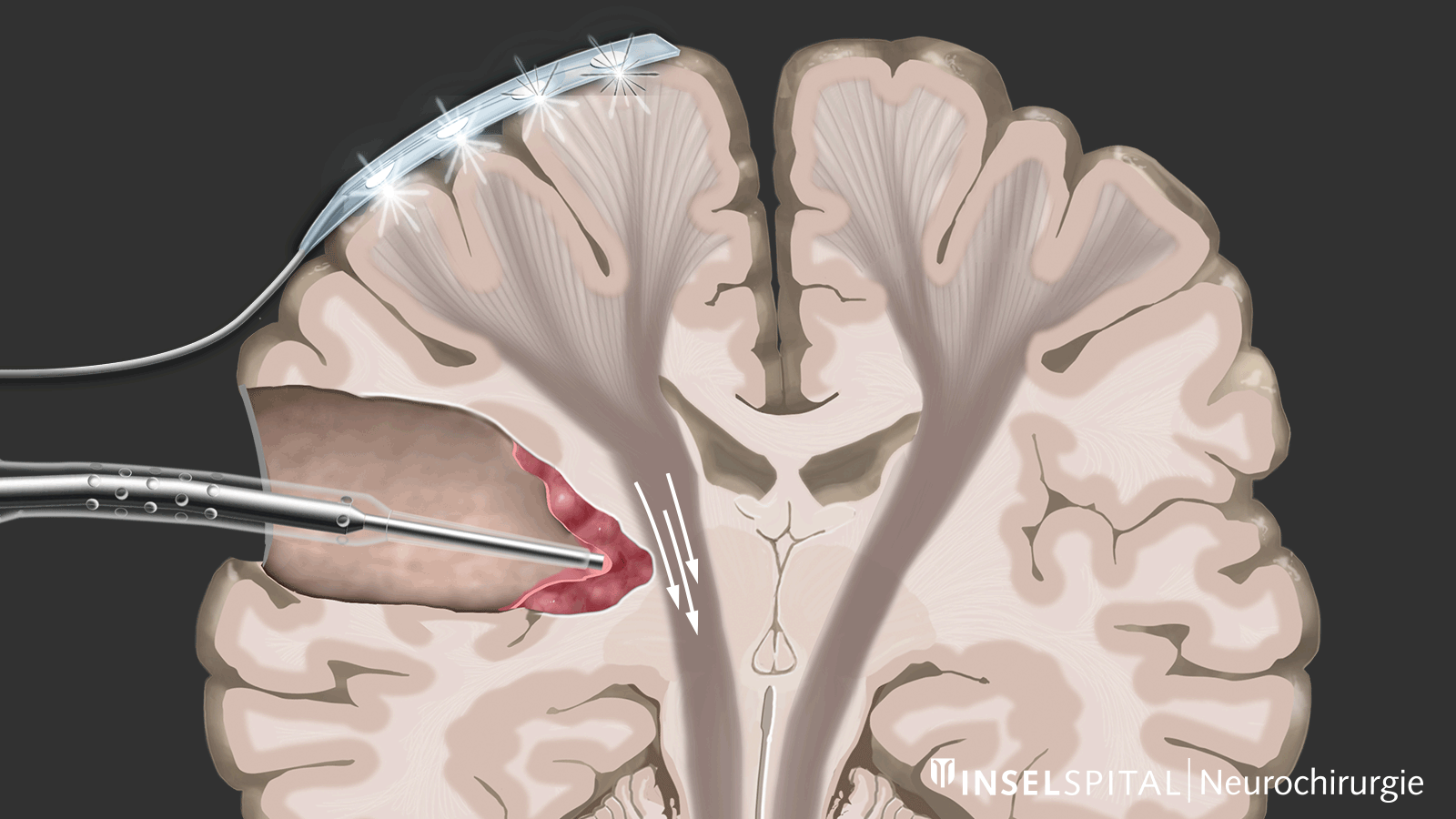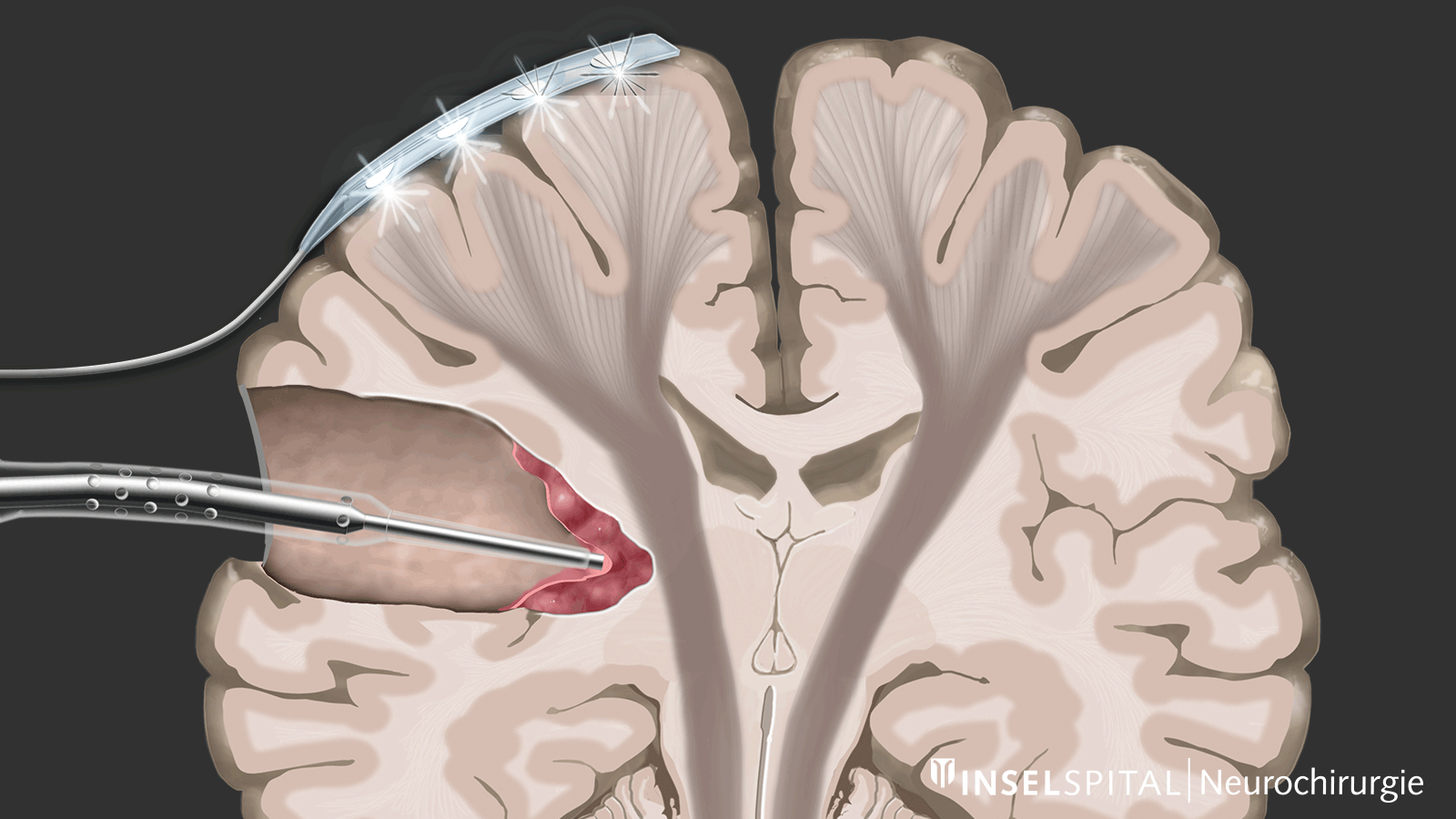
Monitoring is a monitoring method. An electrical impulse is continuously applied via a monitoring electrode placed on the center of movement. This triggers a single movement impulse that is received in the target muscle. Triggering the impulse in the brain and receiving it in the target muscle signal an intact and functioning movement pathway.
Dynamic mapping
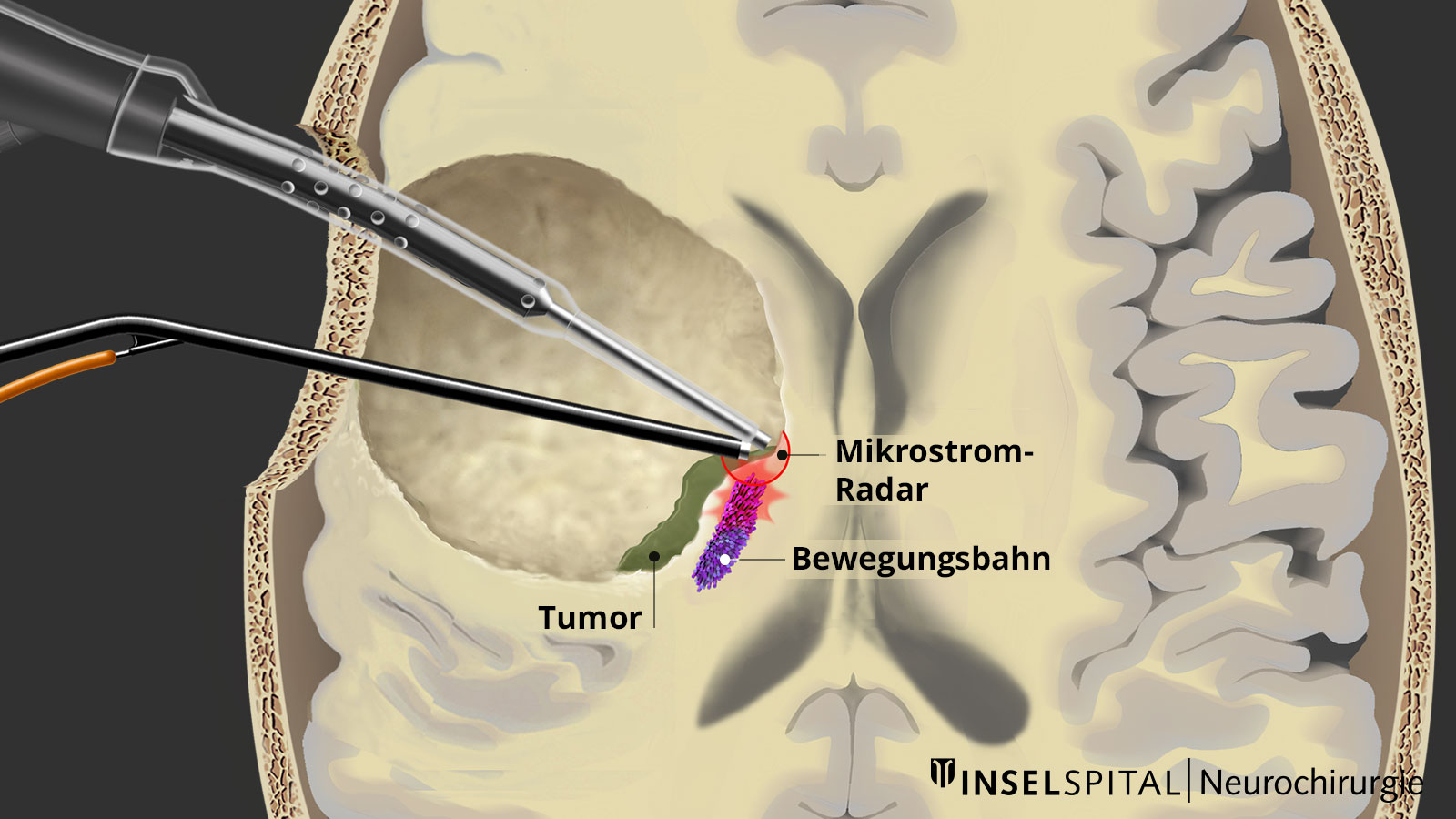
Dynamic mapping is the continuous scanning of brain tissue at the site of tumor removal with a radar of microcurrents. Surgery for glioma should always be performed with specialized functional mapping of speech, movement, vision, and higher brain functions when the tumor location is critical.
Thanks to this procedure and comprehensive intraoperative monitoring, the complication rate for such operations is nowadays very low * * * * * * * *.
Awake surgery
Unlike motor functions, complex functions such as speech, language comprehension, arithmetic, music-making or reading cannot be monitored in patients under anesthesia. Therefore, it is necessary that the patient is awake during part of the surgery and performs special tests together with a neuropsychologist. The location of an invisible brain function can thus be localized and spared during surgery.
5-ALA fluorescence
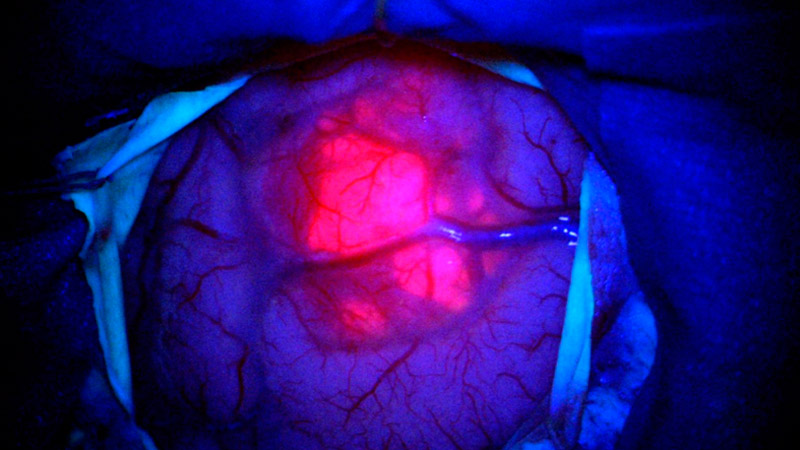
An important component of modern tumor surgery is the routine use of 5-ALA (Gliolan®) *. 5-ALA is administered to patients as a liquid a few hours before the operation. A metabolic product is formed which accumulates in tumor cells of high-grade gliomas and has fluorescent properties *. During the operation under blue light, the surgeon can recognize the tumour as a reddish fluorescent structure and remove the diseased tissue in a more targeted manner *, *, *. Healthy brain tissue shows no fluorescence.
From our own study on fluorescence-assisted resection of glioblastomas, we know that when 5-ALA is used during surgery, the tumor margins extend more than 0.5 cm beyond the tumor volume, which absorbs the contrast medium and is visible in the MRI image *.
Laser Interstitial Thermal Therapy (LITT)
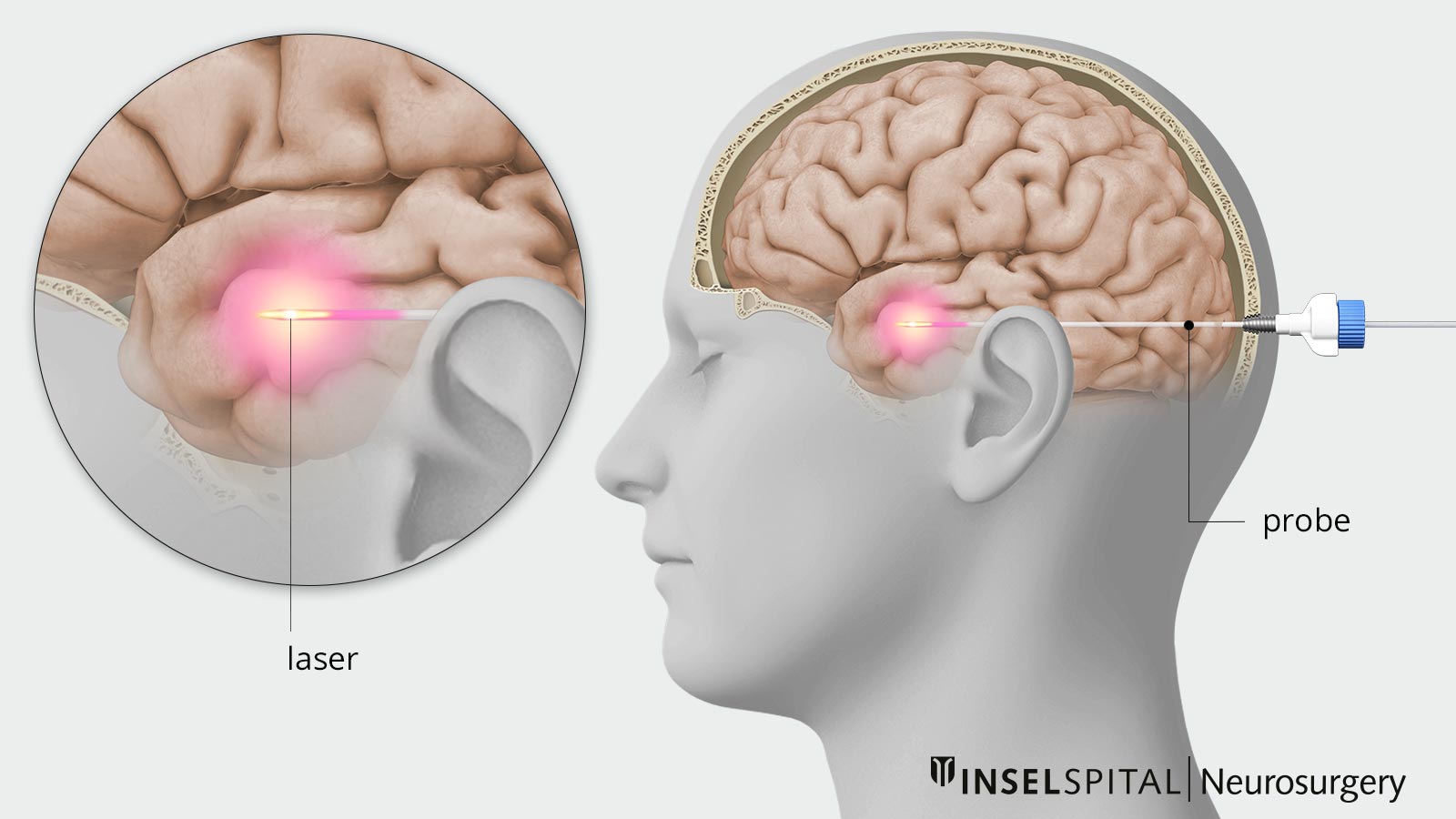
Laser-induced thermotherapy (also known as interstitial laser thermotherapy or LITT for short) is a minimally invasive, image-guided procedure. It uses laser energy to heat and destroy specific tissue. LITT is primarily used to treat epilepsy foci, brain tumors, or other deep-seated changes in the brain.
Intraoperative imaging
Since the distinction between healthy tissue and tumor tissue is difficult even under the microscope, various imaging modalities are used during surgery to directly control the best extent of resection.
Ultrasound is routinely used but has its limitations in visualizing residual tumor tissue.
The most accurate method is intraoperative MRI. For this purpose, after removal of the tumor, the patient is transferred to MRI while the operation is still in progress. In this way, a tumor remnant, if present, can still be removed during the ongoing operation. For this purpose, an operating room with direct connection to a high-field MRI device was built at the Inselspital.
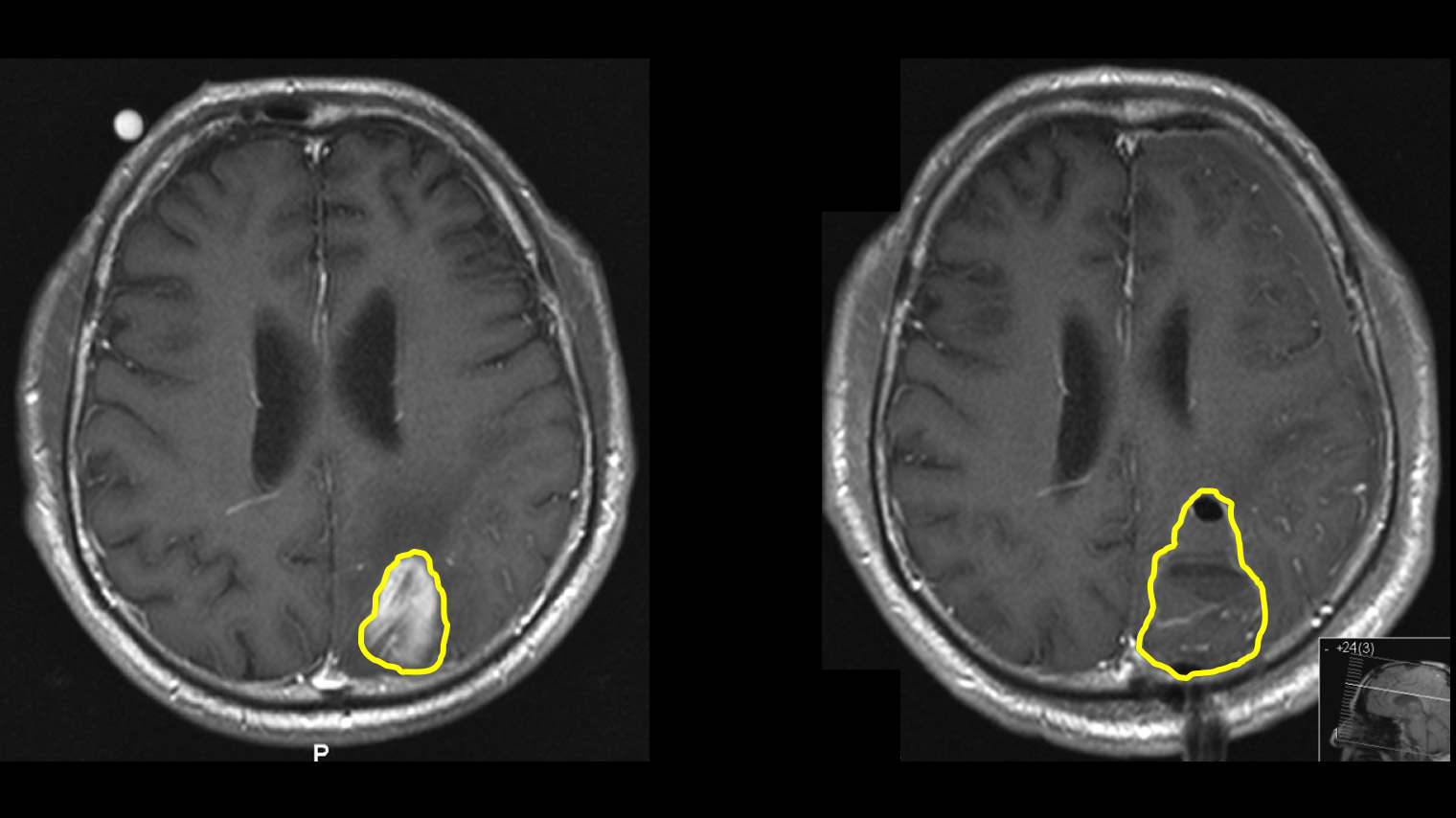
MRI examination 24–48 hours after surgery
Tumor remnants may remain after the operation because the tumor often looks like normal brain tissue in the peripheral area and brain surgery cannot always be performed with a centimeter-wide safety margin around the tumor.
If an early control MRI 24–48 hours after the operation shows that a tiny residual tumor is still visible, this should be removed in a second operation immediately the next day, provided this is possible due to the location. Our experience shows that despite the use of all technical aids, removable residual tumor tissue remains in 5–10% of patients and a second operation is advisable *. In a study of our own patients, we were able to show that the second operation almost always leads to complete removal, is well tolerated and involves only a minimal risk for the patient *. The stay in hospital is only extended by approx. 2 days.
Histology
After the histological examination of the removed tissue, the next steps are discussed by an interdisciplinary team of specialists consisting of neurosurgeons, radiation oncologists and medical oncologists. This takes place at our weekly interdisciplinary tumor board, which is also regularly attended by internationally renowned neuro-oncological experts via video conference.
Neurological rehabilitation
Neurological rehabilitation is useful in cases with neurological deficits, but should not delay follow-up treatment. Patients without neurological deficits generally do not require inpatient rehabilitation.
Radiotherapy and chemotherapy – the next step in treatment
The standard treatment after surgery for patients in a good general condition consists of a combination of chemotherapy and radiotherapy. Radiotherapy and chemotherapy are usually started 3–4 weeks after surgery to give the wound sufficient time to heal *, *.
In radiotherapy, radiation is administered in a total of 30 sessions over 6 weeks. As a rule, there are 5 sessions per week with a dose of 2 Gy each up to a total dose of 60 Gy.
The most common side effects of radiotherapy are
- fatigue
- nausea
- skin irritation
- hair loss
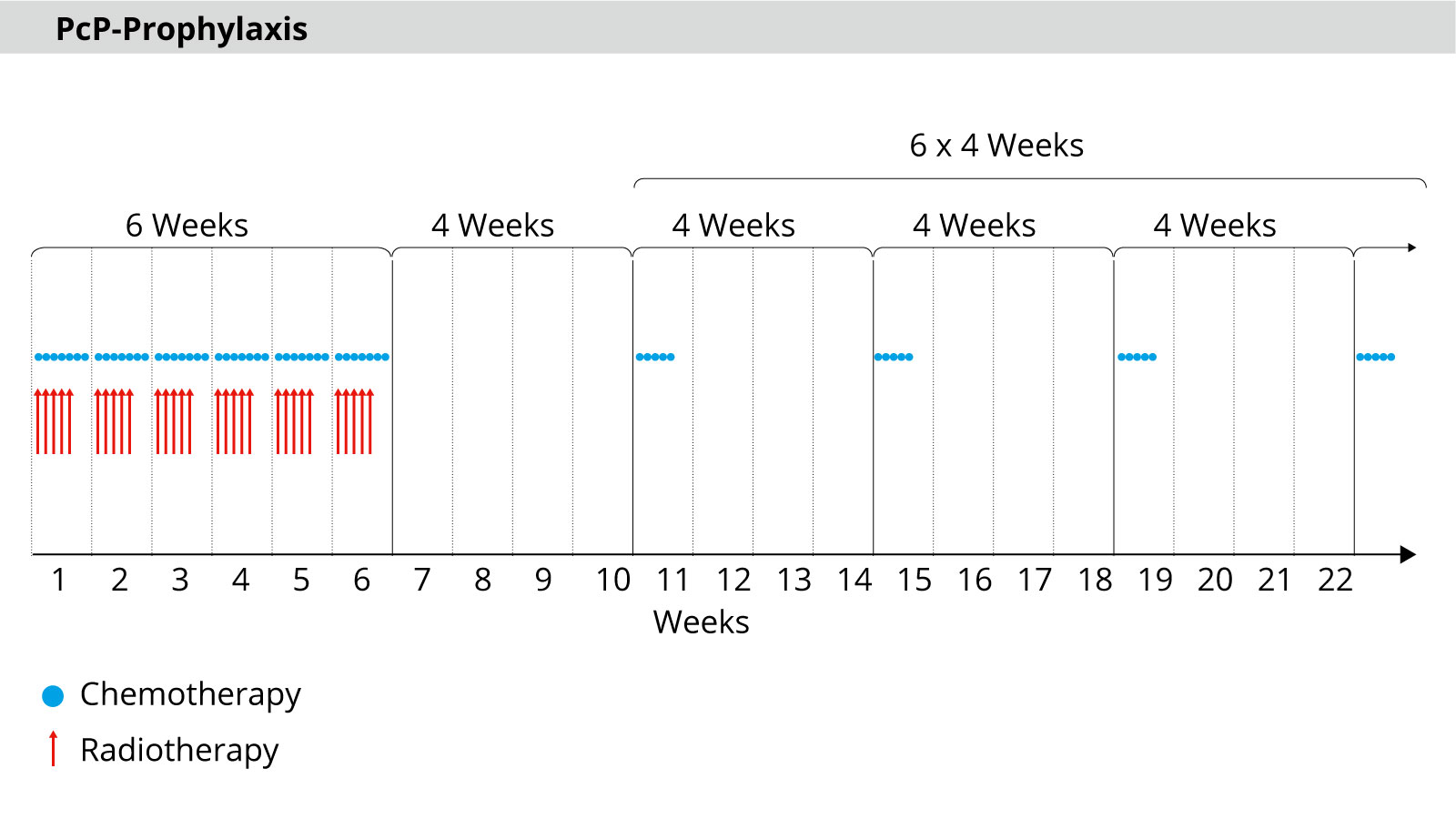
Daily chemotherapy is carried out in parallel with the radiotherapy sessions. After 30 sessions, the maximum possible dose of radiation is reached. Chemotherapy is then continued as maintenance therapy. This means that a cycle of 5 days takes place every 4 weeks – a total of 6 cycles.
Elderly patients
Older patients (over 70 years of age) represent a special case in treatment. In order to avoid putting too much strain on older patients, they are treated according to a shortened schedule. The radiotherapy is then usually carried out in 15 sessions up to a total dose of 40 Gy *.
Alternatively, patients in poorer general health can be treated with either radiation or chemotherapy alone – depending on the DNA of the tumor *.
Temodal
Temozolomide (Temodal®) is used as a chemotherapeutic agent. In the large EORCT-NCIC study, the effectiveness of chemotherapy in addition to radiotherapy was demonstrated *, *. The long-term results of this study also confirm this *. In particular, patients with MGMT promoter methylation, in whom the tumor cells can no longer repair their DNA damage, benefit from additional chemotherapy.
In general, therapy with Temodal is well tolerated. The most common side effect is mild nausea. A rare but severe side effect is bone marrow suppression.
After 6 treatment cycles with Temodal, the patient enters the observation phase with follow-up MRIs every 3 months.
Several trials with longer, intensified or additional Temodal doses did not show the expected better results *, *, *, *.
Lomustine
It is still unclear whether patients with MGMT promoter hypermethylation benefit from the addition of the drug lomustine in addition to radiotherapy and temodal therapy. In the NOA-09 study published in 2019, a survival benefit was found for the combination treatment, but due to the small number of patients, these results are not yet conclusive *.
We treat patients with clear MGMT methylation and a good general condition according to the lomustine/temodal regimen.
Immunotherapy
Immunotherapy represents a promising therapeutic approach. By modulating the immune system, the patient's own immune response against the cancer cells is strengthened. Immunotherapy in the broader sense encompasses several therapeutic approaches:
Peptide vaccination
A type of vaccination is used to sensitize the body's own immune system to specific surface molecules of the glioblastoma cells. Recognition of these surface molecules triggers the immune system's attack on the tumor cells.
Previous large-scale studies, such as with the EGFR-specific peptide vaccine rindopepimut, have been unsuccessful *. A current study on this topic is the NOA-21 study, which is investigating the effect of an IDH1 peptide vaccine alone or in combination with an immune checkpoint inhibitor in IDH1-mutated tumors.
Oncolytic viruses
These viruses preferentially infect tumor cells. Infection with the virus causes the tumor cell to die or become vulnerable to attack by the immune system. Encouraging results have been obtained for glioblastomas with recombinant polioviruses (PVSRIPO), adenoviruses (DNX-2401) and TOCA 511 *, *, *.
Radioimmunotherapy
Here, a radiating radionuclide is coupled with a glioma-specific antibody. The antibody causes the radiating radionuclides to adhere to the glioma cells and thus achieve a high local dose. Tests with the isotopes iodine-131 and yttrium-90 showed encouraging results *.The approach is being pursued further in the current NOA-22 study.
Immune checkpoint inhibitors
This principle is based on the inhibition of checkpoints of the body's own immune system. These checkpoints normally inhibit the immune response and prevent uncontrolled activation of the immune system. Some tumors can specifically activate these checkpoints and thus escape the immune system. Therefore, therapy with checkpoint inhibitors is aimed at suppressing these checkpoints and consequently activating the immune system. The best-studied checkpoint is the interaction between PD-1 and PD-1 ligand. Antibodies against PD-1, such as nivolumab, durvalumab or avelumab, are successfully used in various types of cancer and are also being tested in glioblastoma. The NOA-21 study is currently testing avelumab with or without combination with a peptide vaccine.
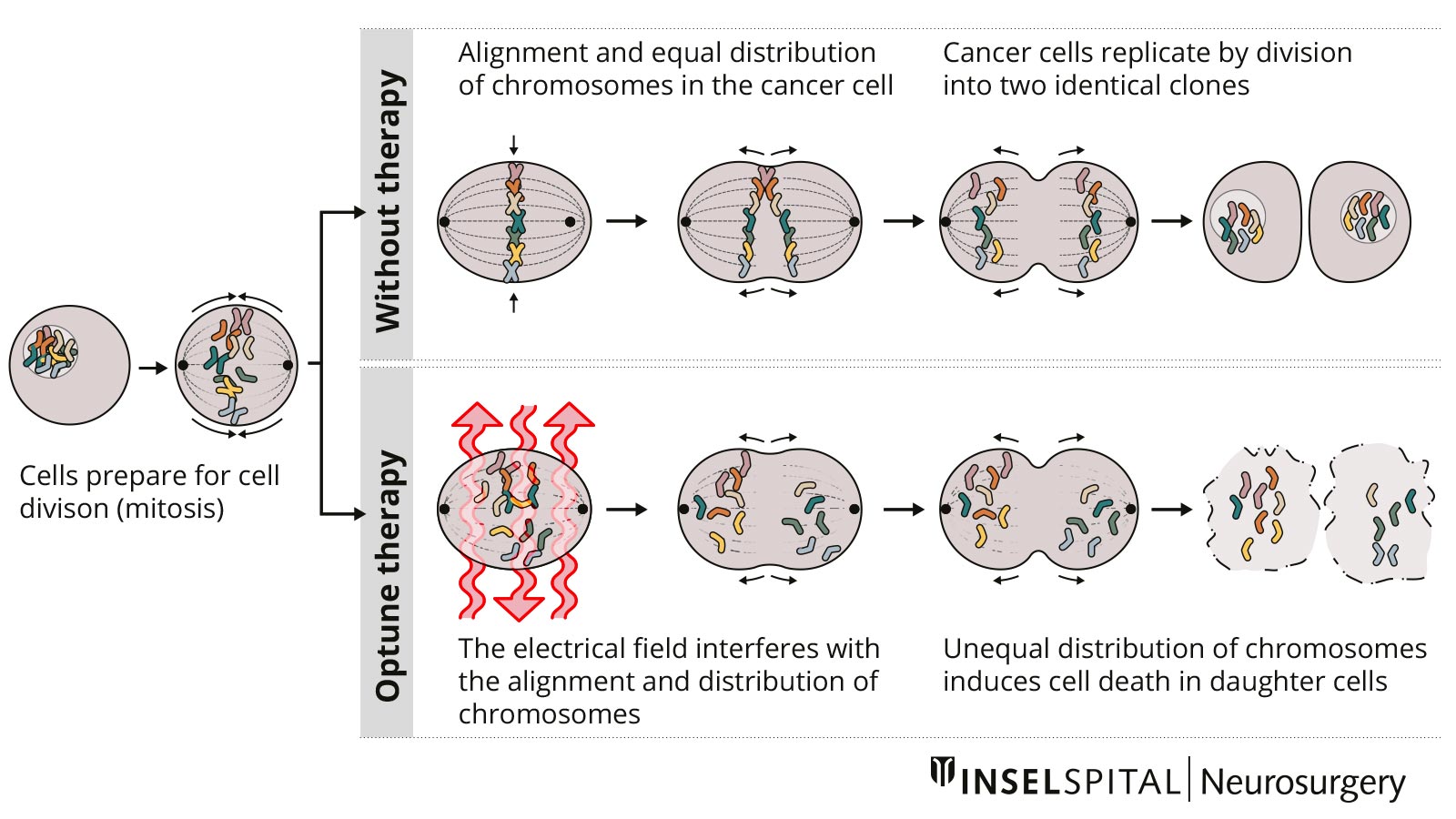
Tumor treating fields
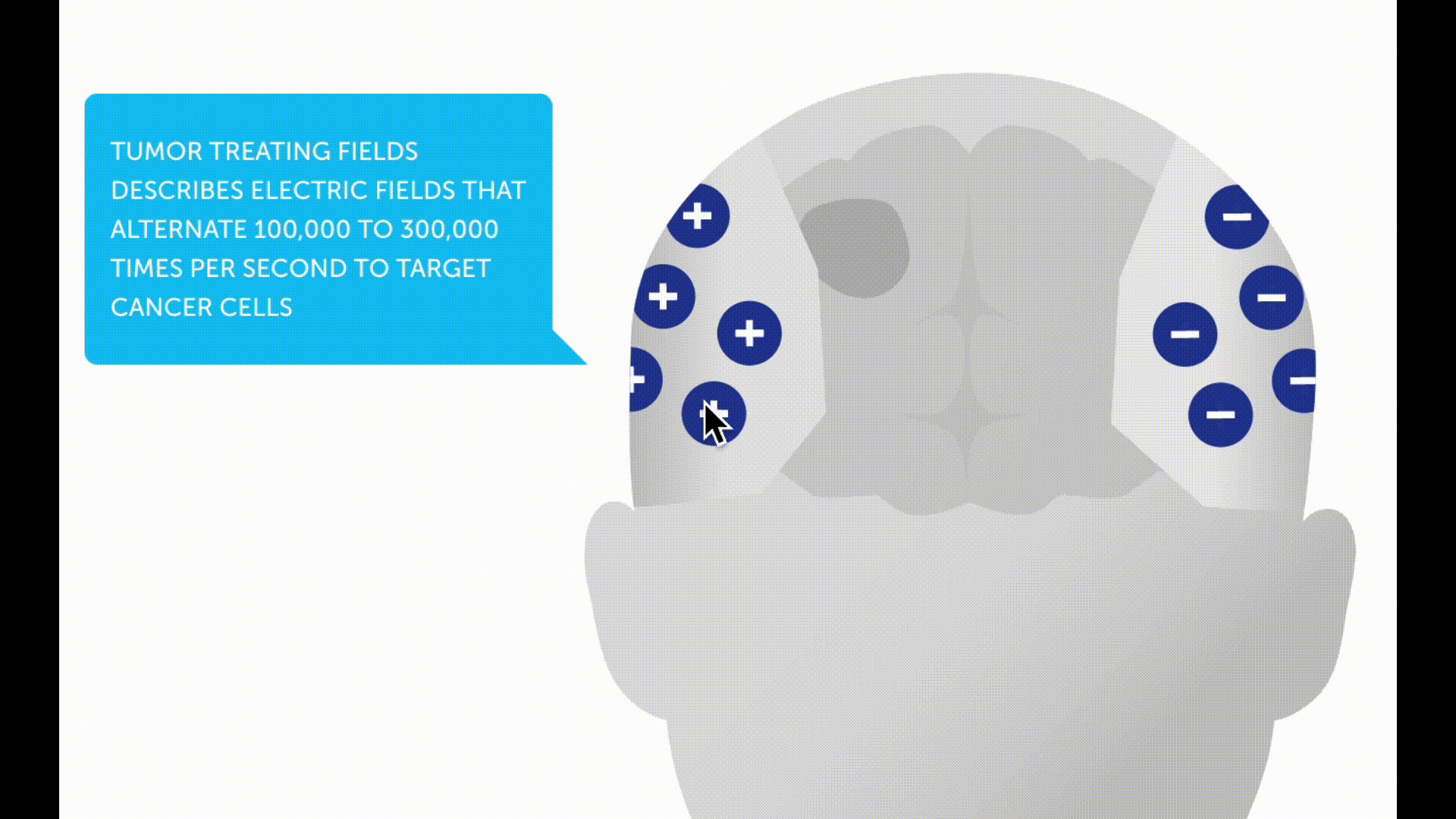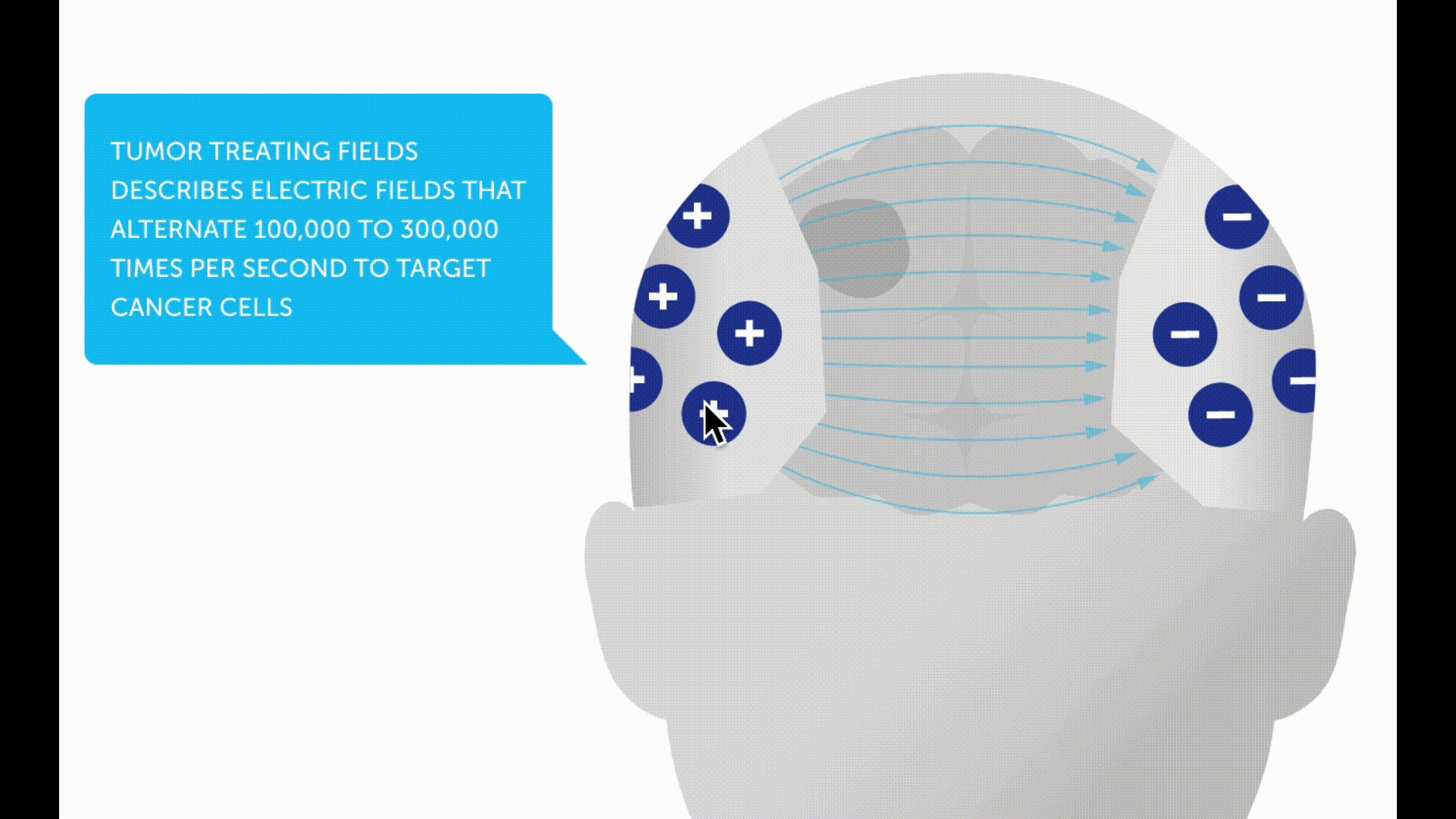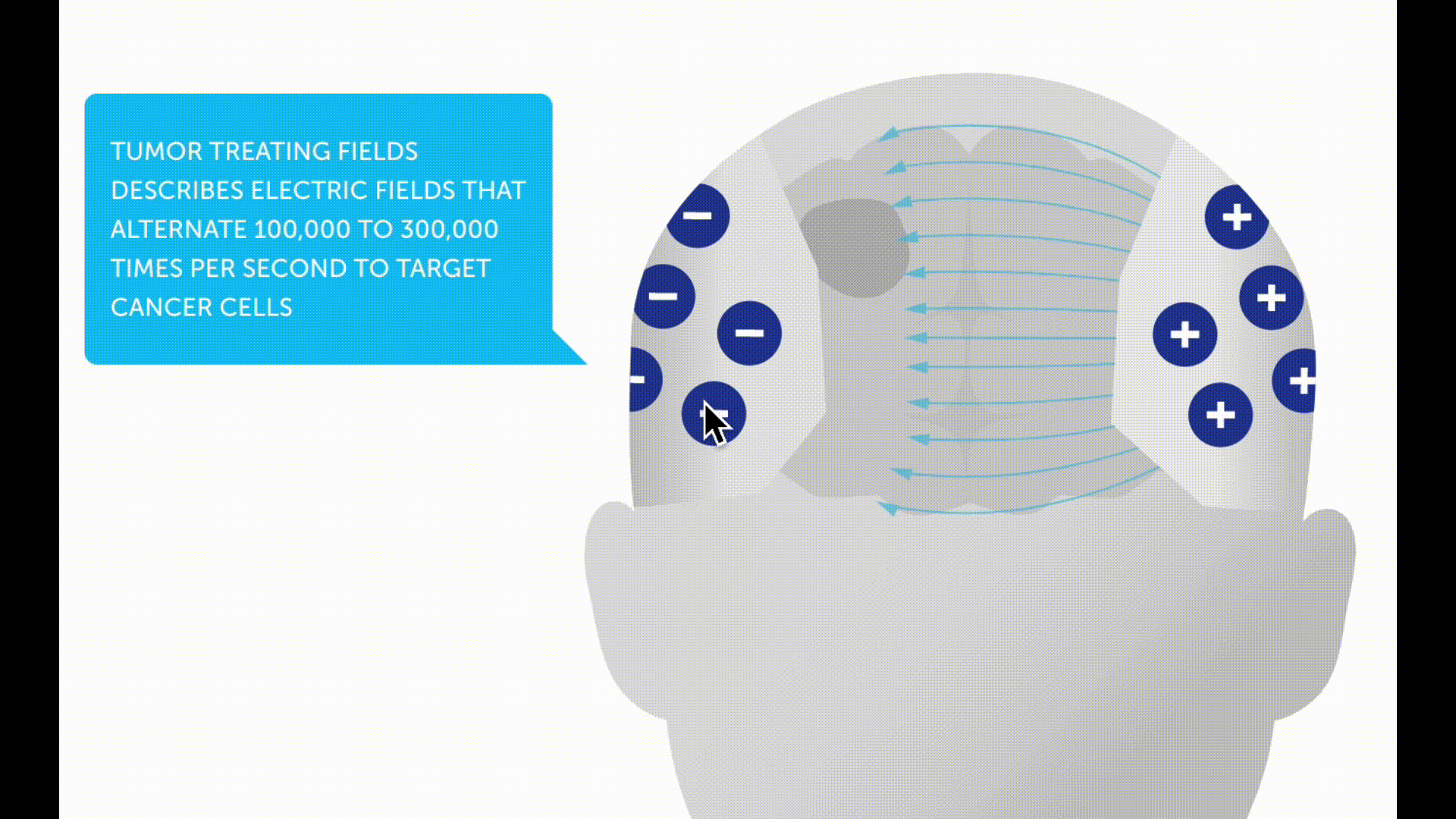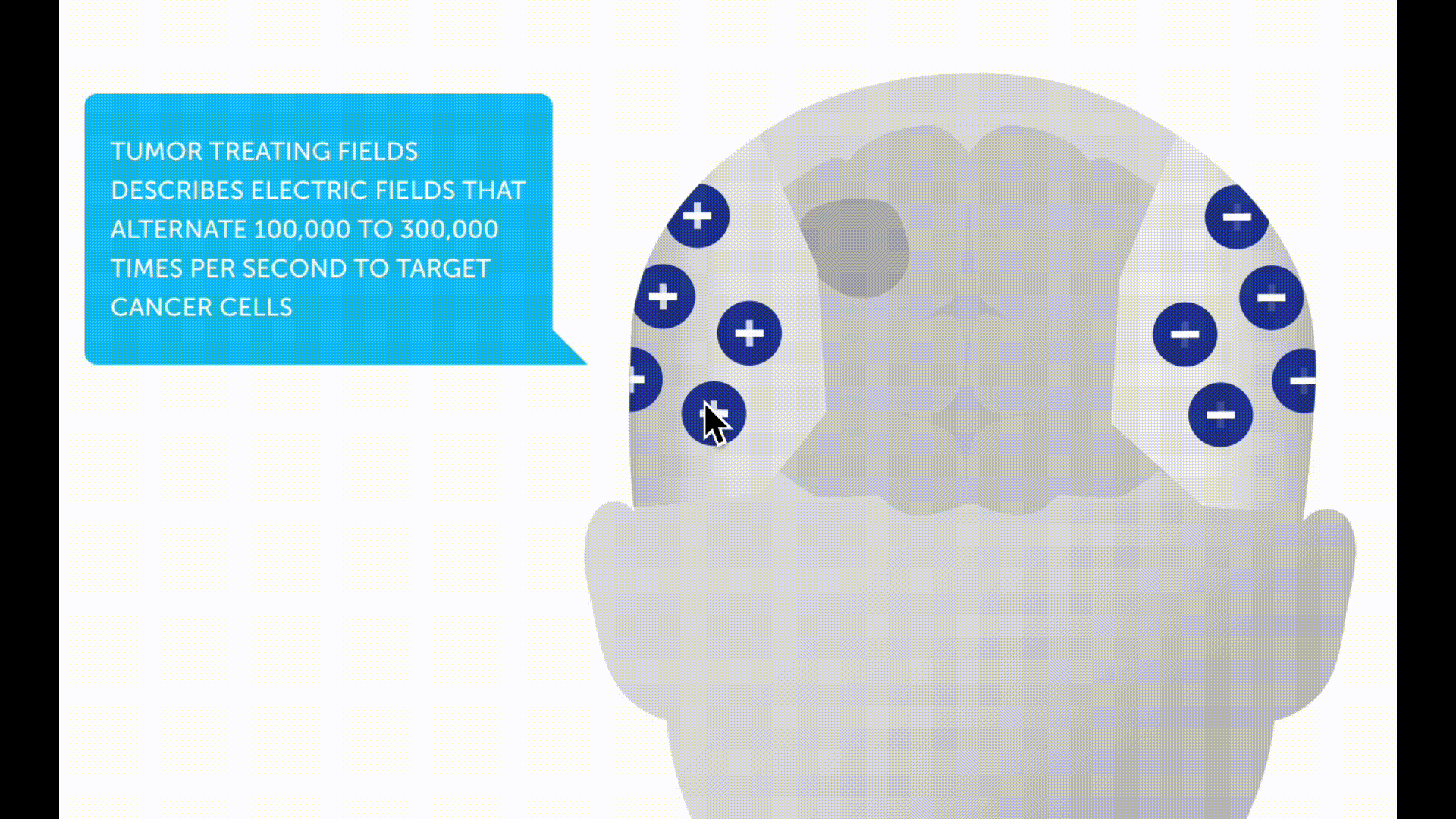
Tumor treating fields (TTFs) are a new non-invasive therapy method for patients with glioblastoma. A therapy device (Optune®) is used to generate low-intensity electric fields, known as tumor treating fields, via a transducer placed on the scalp. These are intended to prevent tumor cells from dividing and multiplying.
In the case of a newly diagnosed glioblastoma, a study with alternating current fields showed a survival advantage for patients who were treated with TTF after radiotherapy in addition to chemotherapy *. The device must be worn daily for as long as possible and the hair must be completely shaved.
We are of the opinion that treatment with TTF will establish itself as the "gold standard" in the treatment of glioblastoma at the latest after a further positive study or with a new form of implantation. We will be happy to answer your questions and put you in touch with us.
Methadone
In recent years, the topic of methadone in the context of glioblastoma therapy has been increasingly discussed. Despite the numerous contributions in internet forums and some reservations about the standard therapy, the assumption that the administration of methadone helps is not based on systematic, scientifically collected and publicly accessible data. To date, there is no evidence of the effectiveness of methadone therapy for glioblastoma. Furthermore, methadone is not without side effects.
What happens if the tumor grows again?
Unfortunately, most patients with a glioblastoma suffer a recurrence at some point, even after successful surgery and subsequent ideal follow-up treatment. One reason for this is the diffuse infiltrating growth of these tumors. On the other hand, there are indications that so-called tumor stem cells play an important role. These may already play a leading role in the development of the tumor, are resistant to the usual therapies and thus trigger the recurrence. Ideally, a therapy should also be effective against these tumor stem cells. Unfortunately, this is not yet the case.
The treatment for a relapse depends on various factors:
- neurological condition of the patient
- temporal dynamics
- molecular tumor markers
- therapies already received
At Inselspital, we take an aggressive approach based on observing the growth pattern. As tumors grow, they create their own environment in which they can thrive better. That is why close MRI monitoring and immediate treatment of even the smallest recurrences is one of our most important principles of the OPTIMISST protocol. We are convinced that a waiting period of several weeks leads to a reduction in survival time, because during this time the tumor sends "refueled" cells into the wider environment that evade surgical resection.
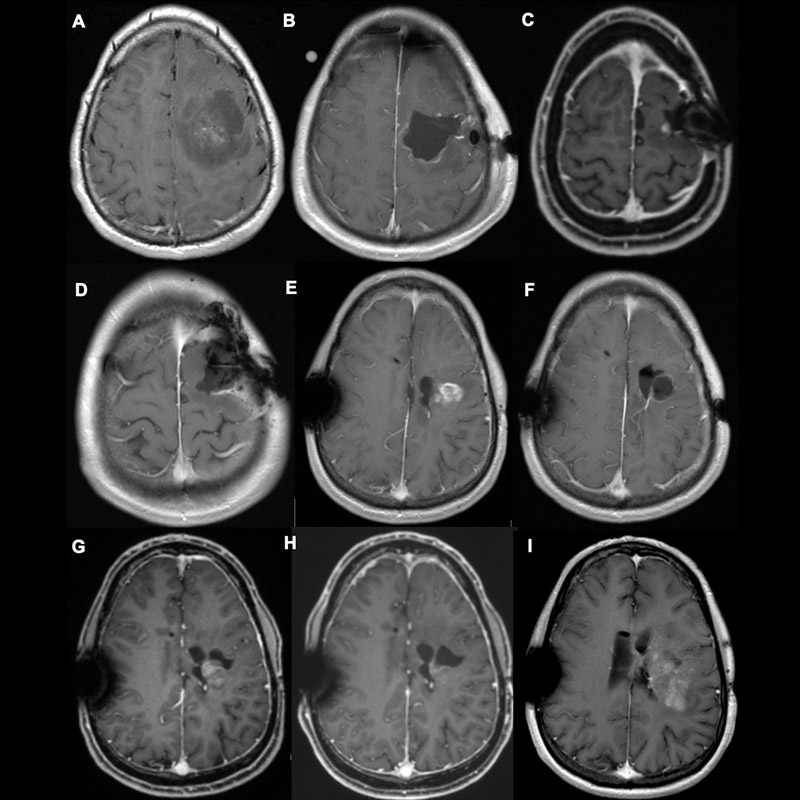
Surgery for a recurrence
In the event of recurrence, surgical resection of the recurrent tumor should always be considered. However, a higher complication rate is to be expected with recurrence operations. In this case, survival time can only be favorably influenced if the functions that ensure a good quality of life for the patient are maintained.
We have therefore initiated the ReSurge study at Inselspital, which is currently being conducted in Europe. We want to find out which patients benefit from recurrence surgery and which do not.
Radiotherapy and/or chemotherapy for a recurrence
In addition to surgery, further radiotherapy and/or chemotherapy can often be carried out. In patients with MGMT promoter hypermethylation and a relapse during the treatment break, the DIRECTOR study was able to prove that these patients benefit again from treatment with Temodal *.
A standardized second-line chemotherapy does not yet exist. We decide on this as part of our interdisciplinary tumor board at the Inselspital. Important factors in the decision are the different molecular characteristics of the tumor and a detailed genetic analysis of the tumor's driving mutations. In discussions with renowned experts, who are connected to the tumor board via conference call, it may then be possible to identify and use drugs that have been successful in other tumor types with the same mutations.
What is the prognosis for glioblastoma?
The prognosis of the disease is determined by many factors. We know from large studies that various factors have an influence on the survival time of patients.
Factors that influence survival time
Factors that cannot be influenced
- younger age
- good general and performance condition
- no loss of neurological functions before the operation
- in the tumor tissue: MGMT promoter hypermethylation of the tumor
- in the tumor tissue: IDH mutation
Factors that can be influenced
- short time to surgery, i.e. earliest possible removal of the tumor
- minimal or no steroid administration (dexamethasone) before and after the operation
- no loss of partial neurological functions after the operation, in particular no paralysis or partial paralysis resulting as a complication of an operation
- complete tumor removal in T1 contrast MRI (CRET according to RANO)
- complete removal of the FLAIR-positive signal in the MRI or the T2-hyperintense signal in the MRI
- no complications during and after the operation
- minimal or no steroid administration (dexamethasone) during radiotherapy
- multifactorial influences (other factors on the OPTIMISST checklist)
It is important to note that only a rough estimate of the course over time can be made for the individual patient, but not an exact prediction. All the figures given are based on the "average value" of a large number of patients and do not allow any conclusions to be drawn about the individual patient.
Long-term survival with glioblastoma under current therapy
Long-term survivors are defined as patients who have survived for more than 5 years after diagnosis. In a large study based on today's standard therapy, this was approximately 10% of patients *. However, this rate varies depending on the characteristics of the patient and the tumor. While it is 17% for patients under 50 years of age, it is only 6.4% for patients over 50 years of age. A young patient age, a good general condition, the presence of an IDH mutation, MGMT promoter hypermethylation and a complete neurosurgical resection have been shown to be associated with long-term survival *, *, *.
Although many long-term survivors have cognitive deficits, a good quality of life can still be achieved in most cases *, *. It is important to know that recurrences also occur in long-term survivors, therefore it is not possible to speak of a cure *.
Why you should seek treatment at Inselspital
At Inselspital, an individual treatment strategy is determined for each patient based on their personal requirements.
This takes place in the certified Brain Tumor Center, where an interdisciplinary team discusses and determines all treatment options individually for each patient. We are certified as an interdisciplinary brain tumor center according to the criteria of the ISO standards (by the international certification institute ClarCert) and the German Cancer Society (by the independent certification institute OnkoZert). All disciplines involved in the tumor board must meet high quality standards.
Our weekly tumor board is interdisciplinary and is made up of specialists from neurosurgery, neurology, neuro-oncology, neuroradiology, nuclear medicine, radio-oncology and pathology.
A supplementary treatment concept, our OPTIMISST protocol, has been developed and implemented at Inselspital.
-
McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65:463-470.
-
Pitter KL, Tamagno I, Alikhanyan K et al. Corticosteroids compromise survival in glioblastoma. Brain. 2016;139:1458-1471.
-
Ostrom QT, Gittleman H, Xu J et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009-2013. Neuro Oncol. 2016;18:v1-v75.
-
Ostrom QT, Gittleman H, de Blank PM et al. American brain tumor association adolescent and young adult primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro-oncology. 2016;18:i1-i50.
-
Lahkola A, Auvinen A, Raitanen J et al. Mobile phone use and risk of glioma in 5 North European countries. International journal of cancer. 2007;120:1769-1775.
-
Ostrom QT, Bauchet L, Davis FG et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16:896-913.
-
Capper D, Jones DTW, Sill M et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555:469-474.
-
Soffietti R, Baumert BG, Bello L et al. Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur J Neurol. 2010;17:1124-1133.
-
Inskip PD, Tarone RE, Hatch EE et al. Cellular-telephone use and brain tumors. N Engl J Med. 2001;344:79-86.
-
Pedersen CL, Romner B. Current treatment of low grade astrocytoma: a review. Clin Neurol Neurosurg. 2013;115:1-8.
-
Claus EB, Black PM. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973-2001. Cancer. 2006;106:1358-1363.
-
Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492-507.
-
Mintz A, Perry J, Spithoff K, Chambers A, Laperriere N. Management of single brain metastasis: a practice guideline. Curr Oncol. 2007;14:131-143.
-
Patchell RA, Tibbs PA, Walsh JW et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494-500.
-
Jakola AS, Skjulsvik AJ, Myrmel KS et al. Surgical resection versus watchful waiting in low-grade gliomas. Ann Oncol. 2017;28:1942-1948.
-
Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115:3-8.
-
Sanai N, Berger MS. Extent of resection influences outcomes for patients with gliomas. Rev Neurol (Paris). 2011;167:648-654.
-
Cancer GARN. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061-1068.
-
Lacroix M, Abi-Said D, Fourney DR et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190-198.
-
Salvati M, Pichierri A, Piccirilli M et al. Extent of tumor removal and molecular markers in cerebral glioblastoma: a combined prognostic factors study in a surgical series of 105 patients. Journal of neurosurgery. 2012;117:204-211.
-
Capelle L, Fontaine D, Mandonnet E et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases. Journal of neurosurgery. 2013;118:1157-1168.
-
McGirt MJ, Chaichana KL, Attenello FJ et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63:700-7; author reply 707.
-
Bloch O, Han SJ, Cha S et al. Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg. 2012;117:1032-1038.
-
Chaichana KL, Cabrera-Aldana EE, Jusue-Torres I et al. When gross total resection of a glioblastoma is possible, how much resection should be achieved. World Neurosurg. 2014;82:e257-65.
-
Keles GE, Lamborn KR, Berger MS. Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg. 2001;95:735-745.
-
Oppenlander ME, Wolf AB, Snyder LA et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg. 2014;120:846-853.
-
Schucht P, Murek M, Jilch A et al. Early re-do surgery for glioblastoma is a feasible and safe strategy to achieve complete resection of enhancing tumor. PLoS One. 2013;8:e79846.
-
Schucht P, Knittel S, Slotboom J et al. 5-ALA complete resections go beyond MR contrast enhancement: shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochir (Wien). 2014;156:305-12; discussion 312.
-
Gill BJ, Pisapia DJ, Malone HR et al. MRI-localized biopsies reveal subtype-specific differences in molecular and cellular composition at the margins of glioblastoma. Proc Natl Acad Sci U S A. 2014;111:12550-12555.
-
Jain R, Poisson LM, Gutman D et al. Outcome prediction in patients with glioblastoma by using imaging, clinical, and genomic biomarkers: focus on the nonenhancing component of the tumor. Radiology. 2014;272:484-493.
-
Yordanova YN, Moritz-Gasser S, Duffau H. Awake surgery for WHO Grade II gliomas within “noneloquent” areas in the left dominant hemisphere: toward a “supratotal” resection. Journal of neurosurgery. 2011
-
Gil-Robles S, Duffau H. Surgical management of World Health Organization Grade II gliomas in eloquent areas: the necessity of preserving a margin around functional structures. Neurosurg Focus. 2010;28:E8.
-
Duffau H. Is supratotal resection of glioblastoma in noneloquent areas possible. World Neurosurg. 2014;82:e101-3.
-
Ius T, Angelini E, Thiebaut de Schotten M, Mandonnet E, Duffau H. Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: towards a “minimal common brain”. Neuroimage. 2011;56:992-1000.
-
Duffau H, Taillandier L. New concepts in the management of diffuse low-grade glioma: Proposal of a multistage and individualized therapeutic approach. Neuro Oncol. 2015;17:332-342.
-
De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30:2559-2565.
-
Duffau H, Capelle L, Denvil D, et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg 2003;98:764-78.
-
Duffau H, Peggy Gatignol ST, Mandonnet E, Capelle L, Taillandier L. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. J Neurosurg 2008;109:
-
Keles GE, Lundin DA, Lamborn KR, Chang EF, Ojemann G, Berger MS. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg. 2004;100:369-375.
-
Raabe A, Beck J, Schucht P, Seidel K. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: evaluation of a new method. J Neurosurg. 2014;120:1015-1024.
-
Chacko AG, Thomas SG, Babu KS et al. Awake craniotomy and electrophysiological mapping for eloquent area tumours. Clin Neurol Neurosurg. 2013;115:329-334.
-
Spena G, Garbossa D, Panciani PP, Griva F, Fontanella MM. Purely subcortical tumors in eloquent areas: awake surgery and cortical and subcortical electrical stimulation (CSES) ensure safe and effective surgery. Clin Neurol Neurosurg. 2013;115:1595-1601.
-
Stummer W, Pichlmeier U, Meinel T et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392-401.
-
Stummer W, Stocker S, Wagner S et al. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery. 1998;42:518-25; discussion 525.
-
Pogue BW, Gibbs-Strauss S, Valdés PA, Samkoe K, Roberts DW, Paulsen KD. Review of Neurosurgical Fluorescence Imaging Methodologies. IEEE J Sel Top Quantum Electron. 2010;16:493-505.
-
Hebeda KM, Saarnak AE, Olivo M, Sterenborg HJ, Wolbers JG. 5-Aminolevulinic acid induced endogenous porphyrin fluorescence in 9L and C6 brain tumours and in the normal rat brain. Acta Neurochir (Wien). 1998;140:503-12; discussion 512.
-
Schucht P, Beck J, Abu-Isa J et al. Gross total resection rates in contemporary glioblastoma surgery: results of an institutional protocol combining 5-aminolevulinic acid intraoperative fluorescence imaging and brain mapping. Neurosurgery. 2012;71:927-35; discussion 935.
-
Louvel G, Metellus P, Noel G, et al. Delaying standard combined chemoradiotherapy after surgical resection does not impact survival in newly diagnosed glioblastoma patients. Radiother Oncol 2016;118:9-15.
-
Loureiro LV, Victor ES, Callegaro-Filho D et al. Minimizing the uncertainties regarding the effects of delaying radiotherapy for Glioblastoma: A systematic review and meta-analysis. Radiother Oncol. 2016;118:1-8.
-
Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996.
-
Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459-66.
-
Chinot OL, Wick W, Mason W et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709-722.
-
Schäfer N, Proescholdt M, Steinbach JP et al. Quality of life in the GLARIUS trial randomizing bevacizumab/irinotecan versus temozolomide in newly diagnosed, MGMT-nonmethylated glioblastoma. Neuro Oncol. 2018;20:975-985.
-
Blumenthal DT, Gorlia T, Gilbert MR et al. Is more better? The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma: a secondary analysis of EORTC and NRG Oncology/RTOG. Neuro Oncol. 2017;19:1119-1126.
-
Gilbert MR, Wang M, Aldape KD et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31:4085-4091.
-
Herrlinger U, Tzaridis T, Mack F et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet. 2019;393:678-688.
-
Wick W, Platten M, Meisner C et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13:707-715.
-
Perry JR, Laperriere N, O’Callaghan CJ et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N Engl J Med. 2017;376:1027-1037.
-
Weller M, Butowski N, Tran DD et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18:1373-1385.
-
Desjardins A, Gromeier M, Herndon JE et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N Engl J Med. 2018;379:150-161.
-
Lang FF, Conrad C, Gomez-Manzano C et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J Clin Oncol. 2018;36:1419-1427.
-
Mitchell LA, Lopez Espinoza F, Mendoza D et al. Toca 511 gene transfer and treatment with the prodrug, 5-fluorocytosine, promotes durable antitumor immunity in a mouse glioma model. Neuro Oncol. 2017;19:930-939.
-
Reulen HJ, Poepperl G, Goetz C et al. Long-term outcome of patients with WHO Grade III and IV gliomas treated by fractionated intracavitary radioimmunotherapy. J Neurosurg. 2015;123:760-770.
-
Stupp R, Taillibert S, Kanner AA et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA. 2015;314:2535-2543.
-
Weller M, Tabatabai G, Kästner B et al. MGMT Promoter Methylation Is a Strong Prognostic Biomarker for Benefit from Dose-Intensified Temozolomide Rechallenge in Progressive Glioblastoma: The DIRECTOR Trial. Clin Cancer Res. 2015;21:2057-2064.
-
Gately L, McLachlan SA, Philip J, Ruben J, Dowling A. Long-term survivors of glioblastoma: a closer look. J Neurooncol. 2018;136:155-162.
-
Krex D, Klink B, Hartmann C et al. Long-term survival with glioblastoma multiforme. Brain. 2007;130:2596-2606.
-
Reifenberger G, Weber RG, Riehmer V et al. Molecular characterization of long-term survivors of glioblastoma using genome- and transcriptome-wide profiling. Int J Cancer. 2014;135:1822-1831.
-
Archibald YM, Lunn D, Ruttan LA et al. Cognitive functioning in long-term survivors of high-grade glioma. J Neurosurg. 1994;80:247-253.
-
Steinbach JP, Blaicher HP, Herrlinger U et al. Surviving glioblastoma for more than 5 years: the patient’s perspective. Neurology. 2006;66:239-242.
-
Bähr O, Herrlinger U, Weller M, Steinbach JP. Very late relapses in glioblastoma long-term survivors. J Neurol. 2009;256:1756-1758.